Rapid evolutionary dynamics of an expanding family of meiotic drive factors and their hpRNA suppressors
- PMID: 34862477
- PMCID: PMC8665063
- DOI: 10.1038/s41559-021-01592-z
Rapid evolutionary dynamics of an expanding family of meiotic drive factors and their hpRNA suppressors
Abstract
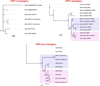
Meiotic drivers are a class of selfish genetic elements whose existence is frequently hidden due to concomitant suppressor systems. Accordingly, we know little of their evolutionary breadth and molecular mechanisms. Here, we trace the evolution of the Dox meiotic drive system in Drosophila simulans, which affects male-female balance (sex ratio). Dox emerged via stepwise mobilization and acquisition of multiple D. melanogaster gene segments including from protamine, which mediates compaction of sperm chromatin. Moreover, we reveal novel Dox homologs and massive amplification of Dox superfamily genes on X chromosomes of its closest sisters D. mauritiana and D. sechellia. Emergence of Dox loci is tightly associated with 359-class satellite repeats that flank de novo genomic copies. In concert, we find coordinated diversification of autosomal hairpin RNA-class siRNA loci that target subsets of Dox superfamily genes. Overall, we reveal fierce genetic arms races between meiotic drive factors and siRNA suppressors associated with recent speciation.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures








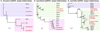
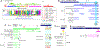





Comment in
-
A flurry of sex-ratio distorters.Nat Ecol Evol. 2021 Dec;5(12):1574-1575. doi: 10.1038/s41559-021-01601-1. Nat Ecol Evol. 2021. PMID: 34862476 No abstract available.
References
-
- Lindholm AK et al. The Ecology and Evolutionary Dynamics of Meiotic Drive. Trends Ecol Evol 31, 315–326 (2016). - PubMed
-
- Jaenike J Sex chromosome meiotic drive. Annu Rev Ecol Syst 32, 25–49 (2001).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

