Models and methods to characterise levonorgestrel release from intradermally administered contraceptives
- PMID: 34862590
- PMCID: PMC8724103
- DOI: 10.1007/s13346-021-01091-5
Models and methods to characterise levonorgestrel release from intradermally administered contraceptives
Abstract
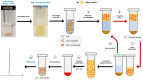
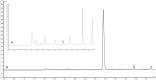
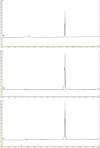
Microneedle (MN)-based technologies have been proposed as a means to facilitate minimally invasive sustained delivery of long-acting hormonal contraceptives into the skin. Intradermal administration is a new route of delivery for these contraceptives and therefore no established laboratory methods or experimental models are available to predict dermal drug release and pharmacokinetics from candidate MN formulations. This study evaluates an in vitro release (IVR) medium and a medium supplemented with ex vivo human skin homogenate (SH) as potential laboratory models to investigate the dermal release characteristics of one such hormonal contraceptive that is being tested for MN delivery, levonorgestrel (LNG), and provides details of an accompanying novel two-step liquid-liquid drug extraction procedure and sensitive reversed-phase HPLC-UV assay. The extraction efficiency of LNG was 91.7 ± 3.06% from IVR medium and 84.6 ± 1.6% from the medium supplemented with SH. The HPLC-UV methodology had a limit of quantification of 0.005 µg/mL and linearity between 0.005 and 25 µg/mL. Extraction and detection methods for LNG were exemplified in both models using the well-characterised, commercially available sustained-release implant (Jadelle®). Sustained LNG release from the implant was detected in both media over 28 days. This study reports for the first time the use of biologically relevant release models and a rapid, reliable and sensitive methodology to determine release characteristics of LNG from intradermally administered long-acting drug delivery systems.
Keywords: Contraception; Intradermal; Levonorgestrel; Method; Microneedles; Skin.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Vrettakos C, Bajaj T. Levonorgestrel. [Updated 2 Mar 2021]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539737/
-
- Gemzell-danielsson K, Berger C, Lalitkumar PG, Berger C, Lalitkumar PG, Gemzell-danielsson K, et al. Mechanisms of action of oral emergency contraception. Gynecol Endocrinol [Internet]. 2014;3590:8–11. Available from: 10.3109/09513590.2014.950648 - PubMed
-
- Graham S, Fraser IS. The progestogen-only mini pill. Contraception [Internet]. 1982;26(4):373–88. Available from: 10.1016/0010-7824(82)90104-4 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

