Efficacy and safety of treating chronic nonspecific low back pain with radial extracorporeal shock wave therapy (rESWT), rESWT combined with celecoxib and eperisone (C + E) or C + E alone: a prospective, randomized trial
- PMID: 34863239
- PMCID: PMC8642949
- DOI: 10.1186/s13018-021-02848-x
Efficacy and safety of treating chronic nonspecific low back pain with radial extracorporeal shock wave therapy (rESWT), rESWT combined with celecoxib and eperisone (C + E) or C + E alone: a prospective, randomized trial
Abstract
Background: To investigate whether respectively radial extracoporeal shock wave therapy (rESWT) or a combination of rESWT, celecoxib and eperisone (rESWT + C + E) are superior in reducing pain in patients with chronic nonspecific low back pain (cnsLBP) compared to C + E alone (a standard treatment of this condition in China).
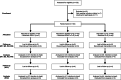
Methods: 140 patients with cnsLBP were randomly allocated to rESWT (n = 47), rESWT + C + E (n = 45) or C + E alone (n = 48) for four weeks between November 2017 and March 2019. Outcome was evaluated using the Pain Self-Efficacy Questionnaire (PSEQ), Numerical Rating Scale (NRS), Oswestry Low Back Pain Disability Questionnaire and Patient Health Questionnaire 9, collected at baseline as well as one week (W1), W2, W3, W4 and W12 after baseline.
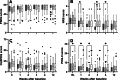
Results: All scores showed a statistically significant improvement over time. The PSEQ and NRS scores showed a significant Time × Treatment effect. Patients treated with rESWT had significantly lower mean NRS values than patients treated with rESWT + C + E at W1 and W3, as well as than patients treated with C + E alone at W3 and W4. No severe adverse events were observed.
Conclusions: rESWT may not be inferior to respectively rESWT + C + E or C + E alone in reducing pain in patients with cnsLBP.
Level of evidence: Level I, prospective, randomized, active-controlled trial.
Trial registration: Clinicaltrials.gov Identifier NCT03337607. Registered November 09, 2017, https://www.clinicaltrials.gov/ct2/show/NCT03337607 .
Level of evidence: Level I; prospective, randomized, controlled trial.
Keywords: Celecoxib; Chronic nonspecific low back pain; Eperisone; Radial extracorporeal shock wave therapy (rESWT).
© 2021. The Author(s).
Conflict of interest statement
This study was performed with the radial extracorporeal shock wave device Swiss DolorClast, which was invented and is manufactured and distributed by Electro Medical Systems (Nyon, Switzerland). CS has received research funding at LMU Munich and consulted (until December 31st, 2017) for Electro Medical Systems. However, Electro Medical Systems had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

