Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden
- PMID: 34863304
- PMCID: PMC8642769
- DOI: 10.1186/s13104-021-05847-7
Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden
Abstract
Objective: Convalescent plasma has been tried as therapy for various viral infections. Early observational studies of convalescent plasma treatment for hospitalized COVID-19 patients were promising, but randomized controlled studies were lacking at the time. The objective of this study was to investigate if convalescent plasma is beneficial to hospitalized patients with COVID-19.
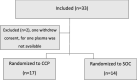
Results: Hospitalized patients with confirmed COVID-19 and an oxygen saturation below 94% were randomized 1:1 to receive convalescent plasma in addition to standard of care or standard of care only. The primary outcome was number of days of oxygen treatment to keep saturation above 93% within 28 days from inclusion. The study was prematurely terminated when thirty-one of 100 intended patients had been included. The median time of oxygen treatment among survivors was 11 days (IQR 6-15) for the convalescent plasma group and 7 days (IQR 5-9) for the standard of care group (p = 0.4, median difference -4). Two patients in the convalescent plasma group and three patients in the standard of care group died (p = 0.64, OR 0.49, 95% CI 0.08-2.79). Thus no significant differences were observed between the groups. Trial registration ClinicalTrials NCT04600440, retrospectively registered Oct 23, 2020.
Keywords: COVID-19; Convalescent plasma; Desaturation; Oxygen therapy; SARS-CoV2.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2014;211(1):80–90. doi: 10.1093/infdis/jiu396. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous