Does the rise in seasonal respiratory viruses foreshadow the return of invasive pneumococcal disease this winter?
- PMID: 34863331
- PMCID: PMC8716133
- DOI: 10.1016/S2213-2600(21)00538-5
Does the rise in seasonal respiratory viruses foreshadow the return of invasive pneumococcal disease this winter?
Conflict of interest statement
CLS, SE, DL, and NKF report payment to their institution for investigator-led research in pneumococcal pneumonia or carriage, or both, in humans by GlaxoSmithKline and Pfizer. All other authors declare no competing interests.
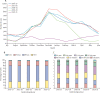
Figures
References
-
- Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3:e360–e370. - PMC - PubMed
-
- Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England. Clin Infect Dis. 2021;72:e65–e75. - PMC - PubMed
-
- Danino D, Ben-Shimol S, van der Beek BA, et al. Decline in pneumococcal disease in young children during the COVID-19 pandemic associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. medRxiv. 2021 doi: 10.1101/2021.07.29.21261308. published online Aug 1. (preprint). - DOI - PMC - PubMed
-
- Ladhani SN, Andrews N, Ramsay ME. Summary of evidence to reduce the two-dose infant priming schedule to a single dose of the 13-valent pneumococcal conjugate vaccine in the national immunisation programme in the UK. Lancet Infect Dis. 2021;21:e93–102. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

