Novel regulatory roles of small leucine-rich proteoglycans in remodeling of the uterine cervix in pregnancy
- PMID: 34863915
- PMCID: PMC9446484
- DOI: 10.1016/j.matbio.2021.11.004
Novel regulatory roles of small leucine-rich proteoglycans in remodeling of the uterine cervix in pregnancy
Retraction in
-
Retraction Notice for "Novel regulatory roles of small leucine-rich proteoglycans in remodeling of the uterine cervix in pregnancy" [Matrix Biology, Volume 105, January 2022, Pages 53-71].Matrix Biol. 2025 Sep;140:154-155. doi: 10.1016/j.matbio.2025.08.001. Epub 2025 Aug 12. Matrix Biol. 2025. PMID: 40849190 No abstract available.
Abstract
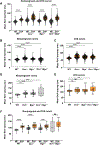
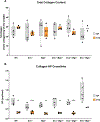
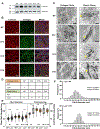
The cervix undergoes rapid and dramatic shifts in collagen and elastic fiber structure to achieve its disparate physiological roles of competence during pregnancy and compliance during birth. An understanding of the structure-function relationships of collagen and elastic fibers to maintain extracellular matrix (ECM) homeostasis requires an understanding of the mechanisms executed by non-structural ECM molecules. Small-leucine rich proteoglycans (SLRPs) play key functions in biology by affecting collagen fibrillogenesis and regulating enzyme and growth factor bioactivities. In the current study, we evaluated collagen and elastic fiber structure-function relationships in mouse cervices using mice with genetic ablation of decorin and/or biglycan genes as representative of Class I SLRPs, and lumican gene representative of Class II SLRP. We identified structural defects in collagen fibril and elastic fiber organization in nonpregnant mice lacking decorin, or biglycan or lumican with variable resolution of defects noted during pregnancy. The severity of collagen and elastic fiber defects was greater in nonpregnant mice lacking both decorin and biglycan and defects were maintained throughout pregnancy. Loss of biglycan alone reduced tissue extensibility in nonpregnant mice while loss of both decorin and biglycan manifested in decreased rupture stretch in late pregnancy. Collagen cross-link density was similar in the Class I SLRP null mice as compared to wild-type nonpregnant and pregnant controls. A broader range in collagen fibril diameter along with an increase in mean fibril spacing was observed in the mutant mice compared to wild-type controls. Collectively, these findings uncover functional redundancy and hierarchical roles of Class I and Class II SLRPs as key regulators of cervical ECM remodeling in pregnancy. These results expand our understating of the critical role SLRPs play to maintain ECM homeostasis in the cervix.
Keywords: Bgn= biglycan; Biomechanics; Cervix; Collagen; D12= gestational day 12; D15= gestational day 15; D18= gestational day 18; D6= gestational day 6; DKO= double knockout; Dcn= decorin; ECM= extracellular matrix; EDS= Ehler-Danlos syndrome; Elastic fibers; Fmod= fibromodulin; HP= hydroxylysylpyridinolines cross-links; LP= lysylpyridinolines cross-links; Lum= lumican; MS= midstroma; Myo= myometrial tissue; NP= non pregnant; PPROM= preterm premature rupture of fetal membranes; PTB= preterm birth; Pregnancy; Proteoglycan; SE= subepithelia; SHG= second harmonic generation imaging; SLRPs= small leucine rich proteoglycans; TEM= tissue electron microscopy; WT= wild-type.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interests The authors declare that they have no conflicts of interest with the contents of this article.
Figures








References
-
- Shynlova O, Mitchell JA, Tsampalieros A, Langille BL, Lye SJ, Progesterone and gravidity differentially regulate expression of extracellular matrix components in the pregnant rat myometrium, Biol Reprod 70(4) (2004) 986–92. - PubMed
-
- Byers PH, Murray ML, Ehlers-Danlos syndrome: a showcase of conditions that lead to understanding matrix biology, Matrix Biol 33 (2014) 10–5. - PubMed
-
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Births: Final Data for 2019, Natl Vital Stat Rep 70(2) (2021) 1–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

