URAT1-selective inhibition ameliorates insulin resistance by attenuating diet-induced hepatic steatosis and brown adipose tissue whitening in mice
- PMID: 34863940
- PMCID: PMC8717577
- DOI: 10.1016/j.molmet.2021.101411
URAT1-selective inhibition ameliorates insulin resistance by attenuating diet-induced hepatic steatosis and brown adipose tissue whitening in mice
Abstract
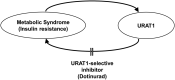
Objective: Accumulating evidence indicates that high uric acid (UA) is strongly associated with obesity and metabolic syndrome and drives the development of nonalcoholic fatty liver disease (NAFLD) and insulin resistance. Although urate transporter-1 (URAT1), which is primarily expressed in the kidneys, plays a critical role in the development of hyperuricemia, its pathophysiological implication in NAFLD and insulin resistance remains unclear. We herein investigated the role and functional significance of URAT1 in diet-induced obese mice.
Methods: Mice fed a high-fat diet (HFD) for 16-18 weeks or a normal-fat diet (NFD) were treated with or without a novel oral URAT1-selective inhibitor (dotinurad [50 mg/kg/day]) for another 4 weeks.
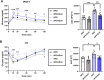
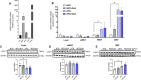
Results: We found that URAT1 was also expressed in the liver and brown adipose tissue (BAT) other than the kidneys. Dotinurad administration significantly ameliorated HFD-induced obesity and insulin resistance. HFD markedly induced NAFLD, which was characterized by severe hepatic steatosis as well as the elevation of serum ALT activity and tissue inflammatory cytokine genes (chemokine ligand 2 (Ccl2) and tissue necrosis factor α (TNFα)), all of which were attenuated by dotinurad. Similarly, HFD significantly increased URAT1 expression in BAT, resulting in lipid accumulation (whitening of BAT), and increased the production of tissue reactive oxygen species (ROS), which were reduced by dotinurad via UCP1 activation.
Conclusions: In conclusion, a novel URAT1-selective inhibitor, dotinurad, ameliorates insulin resistance by attenuating hepatic steatosis and promoting rebrowning of lipid-rich BAT in HFD-induced obese mice. URAT1 serves as a key regulator of the pathophysiology of metabolic syndrome and may be a new therapeutic target for insulin-resistant individuals, particularly those with concomitant NAFLD.
Keywords: Insulin resistance; Metabolic syndrome; NAFLD; URAT1; Uric acid.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures



References
-
- Tanaka Y., Nagoshi T., Yoshii A., Oi Y., Takahashi H., Kimura H., et al. Xanthine oxidase inhibition attenuates doxorubicin-induced cardiotoxicity in mice. Free Radical Biology and Medicine. 2021;162:298–308. - PubMed
-
- Wan X., Xu C., Lin Y., Lu C., Li D., Sang J., et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. Journal of Hepatology. 2016;64(4):925–932. - PubMed
-
- Harmon D.B., Mandler W.K., Sipula I.J., Dedousis N., Lewis S.E., Eckels J.T., et al. Hepatocyte-specific ablation or whole-body inhibition of xanthine oxidoreductase in mice corrects obesity-induced systemic hyperuricemia without improving metabolic abnormalities. Diabetes. 2019;68(6):1221–1229. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

