Cell-cell adhesion impacts epithelia response to substrate stiffness: Morphology and gene expression
- PMID: 34864047
- PMCID: PMC8790207
- DOI: 10.1016/j.bpj.2021.11.2887
Cell-cell adhesion impacts epithelia response to substrate stiffness: Morphology and gene expression
Abstract
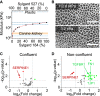
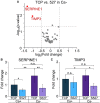
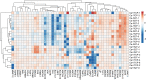
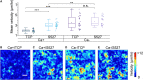
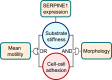
Monolayer epithelial cells interact constantly with the substrate they reside on and their surrounding neighbors. As such, the properties of epithelial cells are profoundly governed by the mechanical and molecular cues that arise from both the substrate and contiguous cell neighbors. Although both cell-substrate and cell-cell interactions have been studied individually, these results are difficult to apply to native confluent epithelia, in which both jointly regulate the cell phenotype. Specifically, it remains poorly understood about the intertwined contributions from intercellular adhesion and substrate stiffness on cell morphology and gene expression, two essential microenvironment properties. Here, by adjusting the substrate modulus and altering the intercellular adhesion within confluent kidney epithelia, we found that cell-substrate and cell-cell interactions can mask each other's influence. For example, we found that epithelial cells exhibit an elongated morphological phenotype only when the substrate modulus and intercellular adhesions are both reduced, whereas their motility can be upregulated by either reduction. These results illustrate that combinatorial changes of the physical microenvironment are required to alter cell morphology and gene expression.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Cellular contractility changes are sufficient to drive epithelial scattering.Exp Cell Res. 2014 Aug 15;326(2):187-200. doi: 10.1016/j.yexcr.2014.04.011. Epub 2014 Apr 26. Exp Cell Res. 2014. PMID: 24780819
-
Characterisation of cell adhesion in airway epithelial cell types using electric cell-substrate impedance sensing.Eur Respir J. 2010 Apr;35(4):894-903. doi: 10.1183/09031936.00065809. Epub 2009 Sep 9. Eur Respir J. 2010. PMID: 19741028
-
Microenvironmental control of premalignant disease: the role of intercellular adhesion in the progression of squamous cell carcinoma.Semin Cancer Biol. 2005 Apr;15(2):84-96. doi: 10.1016/j.semcancer.2004.08.007. Semin Cancer Biol. 2005. PMID: 15652453 Review.
-
Substrate curvature affects the shape, orientation, and polarization of renal epithelial cells.Acta Biomater. 2018 Sep 1;77:311-321. doi: 10.1016/j.actbio.2018.07.019. Epub 2018 Jul 11. Acta Biomater. 2018. PMID: 30006316
-
[Regulation of intercellular adhesion during epithelial morphogenesis].Biol Aujourdhui. 2012;206(3):219-36. doi: 10.1051/jbio/2012021. Epub 2012 Nov 22. Biol Aujourdhui. 2012. PMID: 23171844 Review. French.
Cited by
-
Squishy matters - Corneal mechanobiology in health and disease.Prog Retin Eye Res. 2024 Mar;99:101234. doi: 10.1016/j.preteyeres.2023.101234. Epub 2024 Jan 2. Prog Retin Eye Res. 2024. PMID: 38176611 Free PMC article. Review.
-
Hexanematic crossover in epithelial monolayers depends on cell adhesion and cell density.Nat Commun. 2023 Sep 16;14(1):5762. doi: 10.1038/s41467-023-41449-6. Nat Commun. 2023. PMID: 37717032 Free PMC article.
-
The mechanical influence of densification on epithelial architecture.PLoS Comput Biol. 2024 Apr 1;20(4):e1012001. doi: 10.1371/journal.pcbi.1012001. eCollection 2024 Apr. PLoS Comput Biol. 2024. PMID: 38557605 Free PMC article.
-
Understanding How Cells Probe the World: A Preliminary Step towards Modeling Cell Behavior?Int J Mol Sci. 2023 Jan 23;24(3):2266. doi: 10.3390/ijms24032266. Int J Mol Sci. 2023. PMID: 36768586 Free PMC article.
-
Cyclic mechanical loading of photopolymerized methacrylated hydrogels for probing interdependent effects of strain, stiffness, and substrate composition in pulmonary fibrogenesis.Sci Rep. 2025 Feb 18;15(1):5997. doi: 10.1038/s41598-025-90753-2. Sci Rep. 2025. PMID: 39966483 Free PMC article.
References
-
- Guguen-Guillouzo C., Clément B., et al. Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp. Cell Res. 1983;143:47–54. - PubMed
-
- Proud D., Leigh R. Epithelial cells and airway diseases. Immunol. Rev. 2011;242:186–204. - PubMed
-
- Wilson P.D. Polycystic kidney disease. N. Engl. J. Med. 2004;350:151–164. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

