Substrate-dependent differential regulation of mitochondrial bioenergetics in the heart and kidney cortex and outer medulla
- PMID: 34864090
- PMCID: PMC8957717
- DOI: 10.1016/j.bbabio.2021.148518
Substrate-dependent differential regulation of mitochondrial bioenergetics in the heart and kidney cortex and outer medulla
Abstract
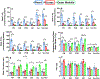
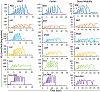
The kinetics and efficiency of mitochondrial oxidative phosphorylation (OxPhos) can depend on the choice of respiratory substrates. Furthermore, potential differences in this substrate dependency among different tissues are not well-understood. Here, we determined the effects of different substrates on the kinetics and efficiency of OxPhos in isolated mitochondria from the heart and kidney cortex and outer medulla (OM) of Sprague-Dawley rats. The substrates were pyruvate+malate, glutamate+malate, palmitoyl-carnitine+malate, alpha-ketoglutarate+malate, and succinate±rotenone at saturating concentrations. The kinetics of OxPhos were interrogated by measuring mitochondrial bioenergetics under different ADP perturbations. Results show that the kinetics and efficiency of OxPhos are highly dependent on the substrates used, and this dependency is distinctly different between heart and kidney. Heart mitochondria showed higher respiratory rates and OxPhos efficiencies for all substrates in comparison to kidney mitochondria. Cortex mitochondria respiratory rates were higher than OM mitochondria, but OM mitochondria OxPhos efficiencies were higher than cortex mitochondria. State 3 respiration was low in heart mitochondria with succinate but increased significantly in the presence of rotenone, unlike kidney mitochondria. Similar differences were observed in mitochondrial membrane potential. Differences in H2O2 emission in the presence of succinate±rotenone were observed in heart mitochondria and to a lesser extent in OM mitochondria, but not in cortex mitochondria. Bioenergetics and H2O2 emission data with succinate±rotenone indicate that oxaloacetate accumulation and reverse electron transfer may play a more prominent regulatory role in heart mitochondria than kidney mitochondria. These studies provide novel quantitative data demonstrating that the choice of respiratory substrates affects mitochondrial responses in a tissue-specific manner.
Keywords: Membrane potential; Mitochondrial bioenergetics; Oxidative phosphorylation; ROS production; Respiration; Reverse electron transport; Substrate metabolism.
Copyright © 2021. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

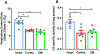






Similar articles
-
Effects of ROS pathway inhibitors and NADH and FADH2 linked substrates on mitochondrial bioenergetics and ROS emission in the heart and kidney cortex and outer medulla.Arch Biochem Biophys. 2023 Aug;744:109690. doi: 10.1016/j.abb.2023.109690. Epub 2023 Jul 8. Arch Biochem Biophys. 2023. PMID: 37429534 Free PMC article.
-
Substrate- and Calcium-Dependent Differential Regulation of Mitochondrial Oxidative Phosphorylation and Energy Production in the Heart and Kidney.Cells. 2021 Dec 31;11(1):131. doi: 10.3390/cells11010131. Cells. 2021. PMID: 35011693 Free PMC article.
-
Computational Modeling of Substrate-Dependent Mitochondrial Respiration and Bioenergetics in the Heart and Kidney Cortex and Outer Medulla.Function (Oxf). 2023 Jul 25;4(5):zqad038. doi: 10.1093/function/zqad038. eCollection 2023. Function (Oxf). 2023. PMID: 37575476 Free PMC article.
-
Role of substrates in the regulation of mitochondrial function in situ.IUBMB Life. 2001 Sep-Nov;52(3-5):221-9. doi: 10.1080/15216540152846037. IUBMB Life. 2001. PMID: 11798036 Review.
-
Mitochondrial membrane lipids in the regulation of bioenergetic flux.Cell Metab. 2024 Sep 3;36(9):1963-1978. doi: 10.1016/j.cmet.2024.07.024. Epub 2024 Aug 22. Cell Metab. 2024. PMID: 39178855 Free PMC article. Review.
Cited by
-
Mitochondrial reactive oxygen species production in lungs of rats with different susceptibilities to hyperoxia-induced acute lung injury.Biochim Biophys Acta Bioenerg. 2025 Jun 12;1866(4):149561. doi: 10.1016/j.bbabio.2025.149561. Online ahead of print. Biochim Biophys Acta Bioenerg. 2025. PMID: 40516644
-
Long-Chain and Medium-Chain Fatty Acids in Energy Metabolism of Murine Kidney Mitochondria.Int J Mol Sci. 2022 Dec 26;24(1):379. doi: 10.3390/ijms24010379. Int J Mol Sci. 2022. PMID: 36613826 Free PMC article.
-
Mitochondria-associated membrane protein PACS2 maintains right cardiac function in hypobaric hypoxia.iScience. 2023 Mar 5;26(4):106328. doi: 10.1016/j.isci.2023.106328. eCollection 2023 Apr 21. iScience. 2023. PMID: 36968068 Free PMC article.
-
Effects of ROS pathway inhibitors and NADH and FADH2 linked substrates on mitochondrial bioenergetics and ROS emission in the heart and kidney cortex and outer medulla.Arch Biochem Biophys. 2023 Aug;744:109690. doi: 10.1016/j.abb.2023.109690. Epub 2023 Jul 8. Arch Biochem Biophys. 2023. PMID: 37429534 Free PMC article.
-
Hypertension and obesity independently drive hypertrophy and alter mitochondrial metabolism in a mouse model of heart failure with preserved ejection fraction.Physiol Rep. 2024 Sep;12(18):e70072. doi: 10.14814/phy2.70072. Physiol Rep. 2024. PMID: 39776299 Free PMC article.
References
-
- Kunz WS, Different metabolic properties of mitochondrial oxidative phosphorylation in different cell types–important implications for mitochondrial cytopathies, Exp. Physiol 88 (1) (2003) 149–154. - PubMed
-
- Johnson DT, et al., Functional consequences of mitochondrial proteome heterogeneity, Am. J. Physiol. Cell Physiol 292 (2) (2007) C698–C707. - PubMed
-
- Herbers E, et al., Tissue specific differences in mitochondrial DNA maintenance and expression, Mitochondrion 44 (2019) 85–92. - PubMed
-
- Pohjoismäki JL, Goffart S, The role of mitochondria in cardiac development and protection, Free Radic. Biol. Med 106 (2017) 345–354. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

