A two-dose optimum for recombinant S1 protein-based COVID-19 vaccination
- PMID: 34864488
- PMCID: PMC8634073
- DOI: 10.1016/j.virol.2021.11.011
A two-dose optimum for recombinant S1 protein-based COVID-19 vaccination
Erratum in
-
Corrigendum to "A two-dose optimum for recombinant S1 protein-based COVID-19 vaccination" [Virology 566 (2022) 56-59].Virology. 2023 Jan;578:22-23. doi: 10.1016/j.virol.2022.11.007. Epub 2022 Nov 26. Virology. 2023. PMID: 36442339 Free PMC article. No abstract available.
Abstract
Background: Recombinant protein subunit vaccination is considered to be a safe, fast and reliable technique when combating emerging and re-emerging diseases such as coronavirus disease 2019 (COVID-19). Typically, such subunit vaccines require the addition of adjuvants to attain adequate immunogenicity. AS01, which contains adjuvants MPL and saponin QS21, is a liposome-based vaccine adjuvant system that is one of the leading candidates. However, the adjuvant effect of AS01 in COVID-19 vaccines is not well described yet.
Methods: In this study, we utilized a mixture of AS01 as the adjuvant for an S1 protein-based COVID-19 vaccine.
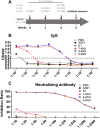
Results: The adjuvanted vaccine induced robust immunoglobulin G (IgG) binding antibody and virus-neutralizing antibody responses. Importantly, two doses induced similar levels of IgG binding antibody and neutralizing antibody responses compared with three doses and the antibody responses weakened only slightly over time up to six weeks after immunization.
Conclusion: These results suggested that two doses may be enough for a clinical vaccine strategy design using MPL & QS21 adjuvanted recombinant protein, especially in consideration of the limited production capacity of COVID-19 vaccine in a public health emergency.
Keywords: Adjuvant; COVID-19; Dose optimum; Protein vaccine.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Didierlaurent A.M., Laupeze B., Di Pasquale A., et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines. 2017;16(1):55–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

