Differentiation therapy for myeloid malignancies: beyond cytotoxicity
- PMID: 34864823
- PMCID: PMC8643352
- DOI: 10.1038/s41408-021-00584-3
Differentiation therapy for myeloid malignancies: beyond cytotoxicity
Abstract
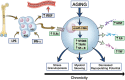
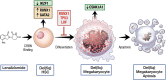
Blocked cellular differentiation is a central pathologic feature of the myeloid malignancies, myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Treatment regimens promoting differentiation have resulted in incredible cure rates in certain AML subtypes, such as acute promyelocytic leukemia. Over the past several years, we have seen many new therapies for MDS/AML enter clinical practice, including epigenetic therapies (e.g., 5-azacitidine), isocitrate dehydrogenase (IDH) inhibitors, fms-like kinase 3 (FLT3) inhibitors, and lenalidomide for deletion 5q (del5q) MDS. Despite not being developed with the intent of manipulating differentiation, induction of differentiation is a major mechanism by which several of these novel agents function. In this review, we examine the new therapeutic landscape for these diseases, focusing on the role of hematopoietic differentiation and the impact of inflammation and aging. We review how current therapies in MDS/AML promote differentiation as a part of their therapeutic effect, and the cellular mechanisms by which this occurs. We then outline potential novel avenues to achieve differentiation in the myeloid malignancies for therapeutic purposes. This emerging body of knowledge about the importance of relieving differentiation blockade with anti-neoplastic therapies is important to understand how current novel agents function and may open avenues to developing new treatments that explicitly target cellular differentiation. Moving beyond cytotoxic agents has the potential to open new and unexpected avenues in the treatment of myeloid malignancies, hopefully providing more efficacy with reduced toxicity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Yates JW, Wallace HJ, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57:485–8. - PubMed
-
- Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE, et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N. Engl J Med. 1979;301:597–9. - PubMed
-
- Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Sanz G, et al. Azacitidine prolongs overall survival and reduces infections and hospitalizations in patients with WHO-defined acute myeloid leukaemia compared with conventional care regimens: an update. Ecancermedicalscience. 2008;2:121. - PMC - PubMed
-
- List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

