Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial
- PMID: 34864943
- PMCID: PMC9464078
- DOI: 10.1093/cid/ciab988
Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial
Erratum in
-
Correction to: Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomized Clinical Trial.Clin Infect Dis. 2023 Feb 8;76(3):560. doi: 10.1093/cid/ciac970. Clin Infect Dis. 2023. PMID: 36617223 Free PMC article. No abstract available.
Abstract
Background: Therapies for refractory cytomegalovirus infections (with or without resistance [R/R]) in transplant recipients are limited by toxicities. Maribavir has multimodal anti-cytomegalovirus activity through the inhibition of UL97 protein kinase.
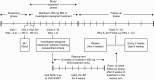
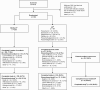
Methods: In this phase 3, open-label study, hematopoietic-cell and solid-organ transplant recipients with R/R cytomegalovirus were randomized 2:1 to maribavir 400 mg twice daily or investigator-assigned therapy (IAT; valganciclovir/ganciclovir, foscarnet, or cidofovir) for 8 weeks, with 12 weeks of follow-up. The primary endpoint was confirmed cytomegalovirus clearance at end of week 8. The key secondary endpoint was achievement of cytomegalovirus clearance and symptom control at end of week 8, maintained through week 16.
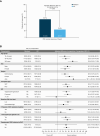
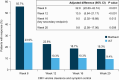
Results: 352 patients were randomized (235 maribavir; 117 IAT). Significantly more patients in the maribavir versus IAT group achieved the primary endpoint (55.7% vs 23.9%; adjusted difference [95% confidence interval (CI)]: 32.8% [22.80-42.74]; P < .001) and key secondary endpoint (18.7% vs 10.3%; adjusted difference [95% CI]: 9.5% [2.02-16.88]; P = .01). Rates of treatment-emergent adverse events (TEAEs) were similar between groups (maribavir, 97.4%; IAT, 91.4%). Maribavir was associated with less acute kidney injury versus foscarnet (8.5% vs 21.3%) and neutropenia versus valganciclovir/ganciclovir (9.4% vs 33.9%). Fewer patients discontinued treatment due to TEAEs with maribavir (13.2%) than IAT (31.9%). One patient per group had fatal treatment-related TEAEs.
Conclusions: Maribavir was superior to IAT for cytomegalovirus viremia clearance and viremia clearance plus symptom control maintained post-therapy in transplant recipients with R/R cytomegalovirus. Maribavir had fewer treatment discontinuations due to TEAEs than IAT. Clinical Trials Registration. NCT02931539 (SOLSTICE).
Keywords: antiviral agents; cytomegalovirus; drug resistance; maribavir; transplant recipients.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. R. K. A.: study grant support: AiCuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire. S. A.: research funding as a scientific expert and site principal investigator: Altona, BioMérieux, Biotest, GlaxoSmithKline, Merck, Merck Sharp & Dohme, Qiagen, Shire, a Takeda company; honoraria for lectures paid to their institution: Biotest, Merck Sharp & Dohme, IQone; support for attending meetings: BioMérieux, Biotest, Quality Control for Molecular Diagnostics; advisory board (unpaid): Quality Control for Molecular Diagnostics. Primary investigator for this study in France. B. D. A.: research funding for work as an investigator: Scynexis, Shire, a Takeda company; research funding to institution for work as an investigator: Astellas, Cidara, F2G, Leadiant; royalties or licenses: UpToDate; consulting fees: Astellas, Scynexis; leadership or fiduciary role: Past President Infectious Diseases Society of America. E. A. B.: research support to their institution: Hologic, Merck, Takeda; scientific medical advisor (unpaid): Merck; Data and Safety Monitoring Board: Amplyx; leadership or fiduciary role: board member (including office holder) American Society of Transplantation. R. F. C.: institutional research grants: AiCuris, Ansun Biopharma, Chimerix, Janssen, Karius, Merck, Novartis, Oxford Immunotec, Pulmotect, Shire, a Takeda company, Viracor; consulting fees: ADMA Biologics, Ansun Biopharma, Janssen, Merck, Molecular Partners, Qiagen, Shire, a Takeda company; honoraria: Genentech, Merck, Oxford Immunotec, Partner Therapeutics, Pulmotect, Shire, a Takeda company; Data Safety Monitoring Board or Advisory Board: Enanta, Duke; stock or stock options: Xenex. C. C.: departmental research funding: Merck, Shire, a Takeda company; consulting fees for advisory board and speaker bureau participation: Merck, Takeda. R. F. D.: departmental research funding: Janssen, Merck, Novartis, Omeros, Roche Diagnostics; consulting fees for advisory boards and speaker bureau participation: Bristol Myers Squibb, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Merck, Omeros, Pfizer, Sanofi Oncology, Sobi, Shire, a Takeda company. D. F. F.: research support for work as an investigator: Astellas, Merck, Nobelpharma, Novavax, Shire, a Takeda company; Data Safety Monitoring Board: Amplyx; advisory boards: Merck, Takeda. N. K.: advisory board and speaker’s fees: Astellas, Biotest, Chiesi, CSL Behring, Merck Sharp & Dohme, Neovii, Novartis Pharma, Sandoz, Sanofi, Shire, a Takeda company. D. K.: consultant: Roche, Sanofi, Takeda; grant/research support: Merck, Qiagen, Roche, Takeda; speaking fee: Astellas. J. M.: consulting fees from Shire/Takeda during the conduct of the study, as well as consulting fees and nonfinancial support from Amgen, Astellas Pharma, Basilea, Cidara, F2G, Schering-Plough, Scynexis; and grants, consulting fees, and nonfinancial support from Bio-Rad, Gilead Sciences, Merck, Pfizer Inc, outside the submitted work; honoraria: Astellas, F2G, Gilead, Pfizer Inc, Merck Sharp & Dohme, Mundipharma. F. M. M.: consultant: AlloVir, Amplyx, Avir, Emcure, F2G, Gilead, Janssen, Kyorin, Merck, Regeneron, ReViral, Symbio, United Medical; investigator and research funding: Ansun, Chimerix, Cidara, Scynexis, Shire, a Takeda company, WHISCON; research funding: AlloVir, Amplyx, F2G, Gilead, Merck, Regeneron. G. A. P.: consulting fees: ADMA Biologics, AlloVir, Amplyx, Astellas, Behring, Cidara, Octapharma, Partner Therapeutics, Shionogi, Shire, a Takeda company, Siemens Healthineers; investigator: Merck, Shire, a Takeda company; honoraria for speaking engagement: Basilea, Merck, Merck Sharp & Dohme Europe. F. P. S.: research support for work as an investigator: Ansun Biopharma, Gilead, Merck, Novartis, Qiagen, Shire, a Takeda company, SlieaGen, WHISCON; travel funding to meetings: Shire, a Takeda company; lecture honoraria: Janssen; consulting fees: Takeda. O. W.: research grants for clinical studies, speaker’s fees, honoraria, and travel expenses: Alexion, Amgen, Astellas, Basilea, Biotest, Bristol Myers Squibb, Chiesi, Correvio, Gilead, Hexal, Janssen, Dr. F. Köhler Chemie, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, Takeda, Teva, UCB; unrestricted grant from the Rudolf-Ackermann-Stiftung (Stiftung für Klinische Infektiologie). J. W., A. K. S., and M. F.: employees of and holding stock/stock options in: Takeda Development Center Americas, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Felipe CR, Ferreira AN, Bessa A, et al. The current burden of cytomegalovirus infection in kidney transplant recipients receiving no pharmacological prophylaxis. J Bras Nefrol 2017; 39:413–23. - PubMed
-
- Beam E, Lesnick T, Kremers W, Kennedy CC, Razonable RR.. Cytomegalovirus disease is associated with higher all-cause mortality after lung transplantation despite extended antiviral prophylaxis. Clin Transplant 2016; 30:270–8. - PubMed

