Ledipasvir/Sofosbuvir for Patients Coinfected With Chronic Hepatitis C and Hepatitis B in Taiwan: Follow-up at 108 Weeks Posttreatment
- PMID: 34864948
- PMCID: PMC9427145
- DOI: 10.1093/cid/ciab971
Ledipasvir/Sofosbuvir for Patients Coinfected With Chronic Hepatitis C and Hepatitis B in Taiwan: Follow-up at 108 Weeks Posttreatment
Abstract
Background: For patients coinfected with hepatitis C virus (HCV) and hepatitis B virus (HBV), HCV treatment with direct-acting antivirals can lead to HBV reactivation. We evaluated HBV reactivation during ledipasvir/sofosbuvir treatment and 108-week follow-up.
Methods: In Taiwan, 111 patients with HCV genotype 1 or 2 and HBV received ledipasvir/sofosbuvir (90mg/400mg) once daily for 12 weeks. HBV virologic reactivation was defined as postbaseline increase in HBV DNA from either less than the lower limit of quantification (LLOQ, 20 IU/mL) to equal to or more than LLOQ or equal to or more than LLOQ to >1 log10 IU/mL. HBV clinical reactivation was HBV virologic reactivation with alanine aminotransferase (ALT) >2× upper limit of normal. Factors associated with development of HBV virologic or clinical reactivation were evaluated with logistic regression analysis.
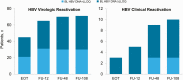
Results: All patients (100%, 111/111) maintained HCV suppression through 108 weeks after treatment. HBV virologic reactivation occurred in 73% of patients (81/111). Clinical reactivation occurred in 9% (10/111). The majority of HBV virologic reactivations (86%, 70/81) occurred by follow-up week 12, whereas clinical reactivation was generally more delayed. Eight (7%, 8/111) initiated HBV therapy. In regression analyses, baseline HBV DNA and hepatitis B surface antigen (HBsAg) levels were associated with HBV virologic reactivation and baseline ALT and HBV DNA, and HBsAg levels were associated with HBV clinical reactivation.
Conclusion: Among HCV/HBV coinfected patients treated with direct-acting antivirals for HCV, HBV virologic reactivation occurred in a majority of patients during treatment and follow-up. In most patients, HBV virologic reactivation was asymptomatic; only a small proportion initiated HBV treatment. Notably, clinical reactivation may still occur >3 months after end of therapy.
Clinical trials registration: NCT02613871.
Keywords: alanine aminotransferase; coinfection; hepatitis B surface antigen; reactivation.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C.-J.L. has served as a consultant for Gilead Sciences, Inc., AbbVie, BMS, and Spring Bank; has given sponsored lectures for Gilead Sciences, Inc., AbbVie, BMS, and MSD; and has received grants from MSD. W.-L.C. has served as a consultant for Gilead Sciences, Inc., AbbVie, BMS, Roche, and PharmaEssentia, and has given sponsored lectures for Gilead Sciences, Inc., AbbVie, BMS, MSD, and Roche. T.-H.H. has served as a consultant for Gilead Sciences, Inc., AbbVie, BMS, and PharmaEssentia; has given sponsored lectures for Gilead Sciences, Inc., AbbVie, BMS, and MSD; and has received grants from Gilead Sciences, Inc. J.Y., B.M., V.S., G.C., D.J., F.Z., and A.G. are employees of Gilead Sciences, Inc., and may hold stock interest in the company. C.-J.C. has given sponsored lectures for Gilead Sciences, Inc., and BMS. P.-J.C. has received grants from Roche, BMS, and J&J; has served as a consultant for BMS, Roche, Bayer, MSD, and Taiha; and has given sponsored lectures for BMS and received honorarium for reviewing grant applications for LDA as a reviewer for Gilead Research Program grant, Liver Disease Asia.
Figures

References
-
- Liu CJ, Liou JM, Chen DS, Chen PJ.. Natural course and treatment of dual hepatitis B virus and hepatitis C virus infections. J Formos Med Assoc 2005; 104:783–91. - PubMed
-
- Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM.. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology 2004; 126:1024–9. - PubMed
-
- Donato F, Boffetta P, Puoti M.. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer 1998; 75:347–54. - PubMed
-
- Stroffolini T, Sagnelli E, Sagnelli C, et al. The burden of HBV infection in HCV patients in Italy and the risk of reactivation under DAA therapy. Dig Liver Dis 2019; 51:434–7. - PubMed

