Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Specific Memory B Cells From Individuals With Diverse Disease Severities Recognize SARS-CoV-2 Variants of Concern
- PMID: 34865053
- PMCID: PMC8922005
- DOI: 10.1093/infdis/jiab585
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Specific Memory B Cells From Individuals With Diverse Disease Severities Recognize SARS-CoV-2 Variants of Concern
Abstract
The unprecedented severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has called for substantial investigations into the capacity of the human immune system to protect against reinfection and keep pace with the evolution of SARS-CoV-2. We evaluated the magnitude and durability of the SARS-CoV-2-specific antibody responses against parental WA-1 SARS-CoV-2 receptor-binding domain (RBD) and a representative variant of concern (VoC) RBD using antibodies from 2 antibody compartments: long-lived plasma cell-derived plasma antibodies and antibodies encoded by SARS-CoV-2-specific memory B cells (MBCs). Thirty-five participants naturally infected with SARS-CoV-2 were evaluated; although only 25 of 35 participants had VoC RBD-reactive plasma antibodies, 34 of 35 (97%) participants had VoC RBD-reactive MBC-derived antibodies. Our finding that 97% of previously infected individuals have MBCs specific for variant RBDs provides reason for optimism regarding the capacity of vaccination, prior infection, and/or both, to elicit immunity with the capacity to limit disease severity and transmission of VoCs as they arise and circulate.
Keywords: COVID-19; antibody; disease severity; limiting dilution assay; memory B-cell; variant of concern.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
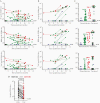
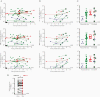

Update of
-
SARS-CoV-2 specific memory B-cells from individuals with diverse disease severities recognize SARS-CoV-2 variants of concern.medRxiv [Preprint]. 2021 Jun 3:2021.05.28.21258025. doi: 10.1101/2021.05.28.21258025. medRxiv. 2021. Update in: J Infect Dis. 2022 Mar 15;225(6):947-956. doi: 10.1093/infdis/jiab585. PMID: 34100028 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

