Neuroinflammatory and Neurometabolomic Consequences From Inhaled Wildfire Smoke-Derived Particulate Matter in the Western United States
- PMID: 34865172
- PMCID: PMC8883349
- DOI: 10.1093/toxsci/kfab147
Neuroinflammatory and Neurometabolomic Consequences From Inhaled Wildfire Smoke-Derived Particulate Matter in the Western United States
Abstract
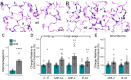
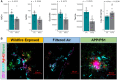
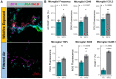
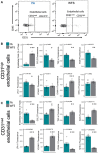
Utilizing a mobile laboratory located >300 km away from wildfire smoke (WFS) sources, this study examined the systemic immune response profile, with a focus on neuroinflammatory and neurometabolomic consequences, resulting from inhalation exposure to naturally occurring wildfires in California, Arizona, and Washington in 2020. After a 20-day (4 h/day) exposure period in a mobile laboratory stationed in New Mexico, WFS-derived particulate matter (WFPM) inhalation resulted in significant neuroinflammation while immune activity in the peripheral (lung, bone marrow) appeared to be resolved in C57BL/6 mice. Importantly, WFPM exposure increased cerebrovascular endothelial cell activation and expression of adhesion molecules (VCAM-1 and ICAM-1) in addition to increased glial activation and peripheral immune cell infiltration into the brain. Flow cytometry analysis revealed proinflammatory phenotypes of microglia and peripheral immune subsets in the brain of WFPM-exposed mice. Interestingly, endothelial cell neuroimmune activity was differentially associated with levels of PECAM-1 expression, suggesting that subsets of cerebrovascular endothelial cells were transitioning to resolution of inflammation following the 20-day exposure. Neurometabolites related to protection against aging, such as NAD+ and taurine, were decreased by WFPM exposure. Additionally, increased pathological amyloid-beta protein accumulation, a hallmark of neurodegeneration, was observed. Neuroinflammation, together with decreased levels of key neurometabolites, reflect a cluster of outcomes with important implications in priming inflammaging and aging-related neurodegenerative phenotypes.
Keywords: VCAM-1; microglia; neuroinflammation; neurovascular unit; particulate matter; smoke.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Agency USEP. (1997). Reference method for the determination of fine particulate matter as PM2.5 in the atmosphere. EPA 40 CFR Part 50, Washington, DC, Appendix L.
-
- Ahmadi Z., Arababadi M. K., Hassanshahi G. (2013). CXCL10 activities, biological structure, and source along with its significant role played in pathophysiology of type I diabetes mellitus. Inflammation 36, 364–371. - PubMed
-
- Aragon M., Erdely A., Bishop L., Salmen R., Weaver J., Liu J., Hall P., Eye T., Kodali V., Zeidler-Erdely P., et al. (2016a). MMP-9-dependent serum-borne bioactivity caused by multiwalled carbon nanotube exposure induces vascular dysfunction via the CD36 scavenger receptor. Toxicol. Sci. 150, 488–498. - PMC - PubMed
-
- Aragon M. J., Topper L., Tyler C. R., Sanchez B., Zychowski K., Young T., Herbert G., Hall P., Erdely A., Eye T., et al. (2017). Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment. Proc. Natl. Acad. Sci. U.S.A. 114, E1968–E1976. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

