Evaluation of the short-term host response and biomechanics of an absorbable poly-4-hydroxybutyrate scaffold in a sheep model following vaginal implantation
- PMID: 34865300
- PMCID: PMC9303173
- DOI: 10.1111/1471-0528.17040
Evaluation of the short-term host response and biomechanics of an absorbable poly-4-hydroxybutyrate scaffold in a sheep model following vaginal implantation
Abstract
Objective: To evaluate the host- and biomechanical response to a fully absorbable poly-4-hydroxybutyrate (P4HB) scaffold in comparison with the response to polypropylene (PP) mesh.
Design: In vivo animal experiment.
Setting: KU Leuven Center for Surgical Technologies.
Population: Fourteen parous female Mule sheep.
Methods: P4HB scaffolds were surgically implanted in the posterior vaginal wall of sheep. The comparative PP mesh data were obtained from an identical study protocol performed previously.
Main outcome measures: Gross necropsy, host response and biomechanical evaluation of explants, and the in vivo P4HB scaffold degradation were evaluated at 60- and 180-days post-implantation. Data are reported as mean ± standard deviation (SD) or standard error of the mean (SEM).
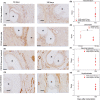
Results: Gross necropsy revealed no implant-related adverse events using P4HB scaffolds. The tensile stiffness of the P4HB explants increased at 180-days (12.498 ± 2.66 N/mm SEM [p =0.019]) as compared to 60-days (4.585 ± 1.57 N/mm) post-implantation, while P4HB degraded gradually. P4HB scaffolds exhibited excellent tissue integration with dense connective tissue and a moderate initial host response. P4HB scaffolds induced a significantly higher M2/M1 ratio (1.70 ± 0.67 SD, score 0-4), as compared to PP mesh(0.99 ± 0.78 SD, score 0-4) at 180-days.
Conclusions: P4HB scaffold facilitated a gradual load transfer to vaginal tissue over time. The fully absorbable P4HB scaffold, in comparison to PP mesh, has a favorable host response with comparable load-bearing capacity. If these results are also observed at longer follow-up in-vivo, a clinical study using P4HB for vaginal POP surgery may be warranted to demonstrate efficacy.
Tweetable abstract: Degradable vaginal P4HB implant might be a solution for treatment of POP.
Keywords: biomechanics; degradable scaffold; host response; pelvic organ prolapse; poly-4-hydroxybutyrate; vaginal surgery.
© 2021 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd.
Conflict of interest statement
CMD, ZG, LH, EV, MZ, EM and JPR declare that they have no competing interests. JD declares that he received a grant from Ethicon (J&J) for an audit of women implanted with Alyte/Ultrapro for abdominal prolapse operations.
Figures







References
-
- Lakeman MM, van der Vaart CH, Laan E, Roovers JPW. The effect of prolapse surgery on vaginal sensibility. J Sex Med. 2011;8(4):1239–45. - PubMed
-
- Weber MA, Lakeman MM, Laan E, Roovers JPW. The effects of vaginal prolapse surgery using synthetic mesh on vaginal wall sensibility, vaginal vasocongestion, and sexual function: a prospective single‐center study. J Sex Med. 2014;11(7):1848–55. - PubMed
-
- Hympanova L, Rynkevic R, Roman S, Mori da Cunha M, Mazza E, Zundel M, et al. Assessment of electrospun and ultra‐lightweight polypropylene meshes in the sheep model for vaginal surgery. Eur Urol Focus. 2020;6(1):190–8. - PubMed
-
- Birch C, Fynes MM. The role of synthetic and biological prostheses in reconstructive pelvic floor surgery. Curr Opin Obstet Gynecol. 2002;14(5):527–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

