Optogenetically controlled human functional motor endplate for testing botulinum neurotoxins
- PMID: 34865655
- PMCID: PMC8647380
- DOI: 10.1186/s13287-021-02665-3
Optogenetically controlled human functional motor endplate for testing botulinum neurotoxins
Abstract
Background: The lack of physiologically relevant and predictive cell-based assays is one of the major obstacles for testing and developing botulinum neurotoxins (BoNTs) therapeutics. Human-induced pluripotent stem cells (hiPSCs)-derivatives now offer the opportunity to improve the relevance of cellular models and thus the translational value of preclinical data.
Methods: We investigated the potential of hiPSC-derived motor neurons (hMNs) optical stimulation combined with calcium imaging in cocultured muscle cells activity to investigate BoNT-sensitivity of an in vitro model of human muscle-nerve system.
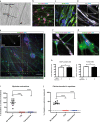
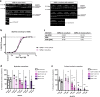
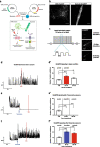
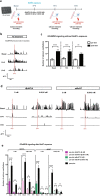
Results: Functional muscle-nerve coculture system was developed using hMNs and human immortalized skeletal muscle cells. Our results demonstrated that hMNs can innervate myotubes and induce contractions and calcium transient in muscle cells, generating an in vitro human motor endplate showing dose-dependent sensitivity to BoNTs intoxication. The implementation of optogenetics combined with live calcium imaging allows to monitor the impact of BoNTs intoxication on synaptic transmission in human motor endplate model.
Conclusions: Altogether, our findings demonstrate the promise of optogenetically hiPSC-derived controlled muscle-nerve system for pharmaceutical BoNTs testing and development.
Keywords: Botulinum neurotoxins; Calcium indicators; Functional; Human-induced pluripotent stem cells; Motor endplate; Optogenetics.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests. CN and JDL are IPSEN employees.
Figures
References
-
- Rossetto O. Botulinum toxins: molecular structures and synaptic physiology. In: Jabbari B, editor. Botulinum toxin treatment in clinical medicine. Cham: Springer International Publishing; 2018. pp. 1–12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

