Both full length-cholesteryl ester transfer protein and exon 9-deleted cholesteryl ester transfer protein promote triacylglycerol storage in cultured hepatocytes
- PMID: 34866179
- PMCID: PMC9060302
- DOI: 10.1002/lipd.12330
Both full length-cholesteryl ester transfer protein and exon 9-deleted cholesteryl ester transfer protein promote triacylglycerol storage in cultured hepatocytes
Abstract
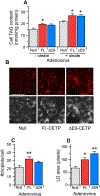
We previously reported that overexpression of full-length cholesteryl ester transfer protein (FL-CETP), but not its exon 9-deleted variant (∆E9-CETP), in an adipose cell line reduces their triacylglycerol (TAG) content. This provided mechanistic insight into several in vivo studies where FL-CETP levels are inversely correlated with adiposity. However, increased FL-CETP is also associated with elevated hepatic lipids, suggesting that the effect of CETP on cellular lipid metabolism may be tissue-specific. Here, we directly investigated the role of FL-CETP and ∆E9-CETP in hepatic lipid metabolism. FL- or ∆E9-CETP was overexpressed in HepG2-C3A by adenovirus transduction. Overexpression of either FL or ∆E9-CETP in hepatocytes increased cellular TAG mass by 25% but reduced TAG secretion. This cellular TAG was contained in larger and more numerous lipid droplets. Analysis of TAG synthetic and catabolic pathways showed that this elevated TAG content was due to increased incorporation of fatty acid into TAG (24%), and higher de novo synthesis of fatty acid (50%) and TAG from acetate (40%). siRNA knockdown of CETP had the opposite effect on TAG synthesis and lipogenesis, and decreased cellular TAG. This novel increase in cellular TAG by FL-CETP overexpression was reproduced in Caco-2 intestinal epithelial cells. We conclude that, unlike that seen in adipocyte cells, overexpression of either CETP isoform in lipoprotein-secreting cells promotes the accumulation of TAG. These data suggest that the in vivo correlation between CETP levels and hepatic steatosis can be explained, in part, by a direct effect of CETP on hepatocyte cellular metabolism.
Keywords: cellular lipid homeostasis; cholesteryl ester transfer protein; hepatocyte; isoforms; lipid metabolism; triacylglycerol.
© 2021 AOCS.
Conflict of interest statement
CONFLICT OF INTEREST
All authors declare that they have no conflicts of interest.
Figures




References
-
- Adams LA, Marsh JA, Ayonrinde OT, Olynyk JK, Ang WQ, Beilin LJ, Mori T, Palmer LJ, Oddy WW, Lye SJ, and Pennell SE. Cholesteryl ester transfer protein gene polymorphisms increase the risk of fatty liver in females independent of adiposity. J Gastroenterol Hepatol. 2012;27:1520–1527. doi:10.1111/j.1440-1746.2012.07120.x. - DOI - PubMed
-
- Barber RD, Harmer DW, A. CR, and Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

