Distribution of androgen receptor mRNA in the prepubertal male and female mouse brain
- PMID: 34866263
- PMCID: PMC8711114
- DOI: 10.1111/jne.13063
Distribution of androgen receptor mRNA in the prepubertal male and female mouse brain
Abstract
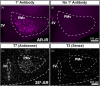
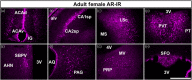
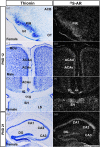
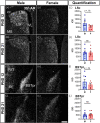
Androgens are steroid hormones that play a critical role in brain development and sexual maturation by acting upon both androgen receptors (AR) and estrogen receptors (ERα/β) after aromatization. The contribution of estrogens from aromatized androgens in brain development and the central regulation of metabolism, reproduction, and behavior is well defined, but the role of androgens acting on AR has been unappreciated. Here, we map the sex specific expression of Ar in the adult and developing mouse brain. Postnatal days (PND) 12 and 21 were used to target a critical window of prepubertal development. Consistent with previous literature in adults, sex-specific differences in Ar expression were most profound in the bed nucleus of the stria terminalis (BST), medial amygdala (MEA) and medial preoptic area (MPO). Ar expression was also high in these areas at PND 12 and 21 in both sexes. In addition, we describe extra-hypothalamic and extra-limbic areas that show moderate, consistent and similar Ar expression in both sexes at both prepubertal time points. Briefly, Ar expression was observed in olfactory areas of the cerebral cortex, the hippocampus, several thalamic nuclei, and cranial nerve nuclei involved in autonomic sensory and motor function. To further characterize forebrain populations of Ar expressing neurons and determine whether they also coexpress estrogen receptors, we examined expression of Ar, Esr1 and Esr2 in prepubertal mice in selected nuclei. We found populations of neurons in the BST, MEA and MPO that coexpress Ar, but not Esr1 or Esr2, whereas others express a combination of the three receptors. Our findings indicate that various brain areas express Ar during prepubertal development and may play an important role in female neuronal development and physiology.
Keywords: gonadal steroids; postnatal development; puberty; sex differences.
© 2021 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
-
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell‐Badge R. Expression of a candidate sex‐determining gene during mouse testis differentiation. Nature. 1990;348(6300):450‐452. - PubMed
-
- Sinclair AH, Berta P, Palmer MS, et al. A gene from the human sex‐determining region encodes a protein with homology to a conserved DNA‐binding motif. Nature. 1990;346(6281):240‐244. - PubMed
-
- Rhoda J, Corbier P, Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17β‐estradiol. Endocrinology. 1984;114(5):1754‐1760. - PubMed
-
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106(1):306‐316. - PubMed
-
- Naftolin F, MacLusky N. Aromatization hypothesis revisited. In: Serio M, ed. Sexual Differentiation: Basic and Clinical Aspects. Serono symposia publications from Raven Press 11. Raven Press; 1984:79‐91.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

