Extracellular Vimentin as a Target Against SARS-CoV-2 Host Cell Invasion
- PMID: 34866333
- PMCID: PMC9252327
- DOI: 10.1002/smll.202105640
Extracellular Vimentin as a Target Against SARS-CoV-2 Host Cell Invasion
Abstract
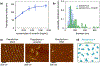
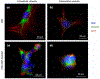
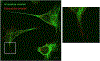
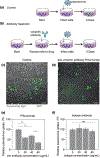
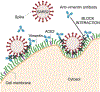
Infection of human cells by pathogens, including SARS-CoV-2, typically proceeds by cell surface binding to a crucial receptor. The primary receptor for SARS-CoV-2 is the angiotensin-converting enzyme 2 (ACE2), yet new studies reveal the importance of additional extracellular co-receptors that mediate binding and host cell invasion by SARS-CoV-2. Vimentin is an intermediate filament protein that is increasingly recognized as being present on the extracellular surface of a subset of cell types, where it can bind to and facilitate pathogens' cellular uptake. Biophysical and cell infection studies are done to determine whether vimentin might bind SARS-CoV-2 and facilitate its uptake. Dynamic light scattering shows that vimentin binds to pseudovirus coated with the SARS-CoV-2 spike protein, and antibodies against vimentin block in vitro SARS-CoV-2 pseudovirus infection of ACE2-expressing cells. The results are consistent with a model in which extracellular vimentin acts as a co-receptor for SARS-CoV-2 spike protein with a binding affinity less than that of the spike protein with ACE2. Extracellular vimentin may thus serve as a critical component of the SARS-CoV-2 spike protein-ACE2 complex in mediating SARS-CoV-2 cell entry, and vimentin-targeting agents may yield new therapeutic strategies for preventing and slowing SARS-CoV-2 infection.
Keywords: SARS-CoV2; cell membranes; endocytosis; extracellular vimentin; pseudoviruses; spike proteins.
© 2021 Wiley-VCH GmbH.
Conflict of interest statement
Competing Interests:
The authors declare no competing interests.
Figures



Update of
-
Extracellular vimentin as a target against SARS-CoV-2 host cell invasion.bioRxiv [Preprint]. 2021 Mar 18:2021.01.08.425793. doi: 10.1101/2021.01.08.425793. bioRxiv. 2021. Update in: Small. 2022 Feb;18(6):e2105640. doi: 10.1002/smll.202105640. PMID: 33442680 Free PMC article. Updated. Preprint.
Similar articles
-
Extracellular vimentin as a target against SARS-CoV-2 host cell invasion.bioRxiv [Preprint]. 2021 Mar 18:2021.01.08.425793. doi: 10.1101/2021.01.08.425793. bioRxiv. 2021. Update in: Small. 2022 Feb;18(6):e2105640. doi: 10.1002/smll.202105640. PMID: 33442680 Free PMC article. Updated. Preprint.
-
Extracellular vimentin is an attachment factor that facilitates SARS-CoV-2 entry into human endothelial cells.Proc Natl Acad Sci U S A. 2022 Feb 8;119(6):e2113874119. doi: 10.1073/pnas.2113874119. Proc Natl Acad Sci U S A. 2022. PMID: 35078919 Free PMC article.
-
Cell surface detection of vimentin, ACE2 and SARS-CoV-2 Spike proteins reveals selective colocalization at primary cilia.Sci Rep. 2022 Apr 29;12(1):7063. doi: 10.1038/s41598-022-11248-y. Sci Rep. 2022. PMID: 35487944 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
-
Exploring Spike Protein as Potential Target of Novel Coronavirus and to Inhibit the Viability Utilizing Natural Agents.Curr Drug Targets. 2021;22(17):2006-2020. doi: 10.2174/1389450122666210309105820. Curr Drug Targets. 2021. PMID: 33687893 Review.
Cited by
-
Mechanobiology in Metabolic Dysfunction-Associated Steatotic Liver Disease and Obesity.Curr Issues Mol Biol. 2024 Jul 7;46(7):7134-7146. doi: 10.3390/cimb46070425. Curr Issues Mol Biol. 2024. PMID: 39057066 Free PMC article. Review.
-
SARS-CoV-2: Receptor and Co-receptor Tropism Probability.Curr Microbiol. 2022 Mar 16;79(5):133. doi: 10.1007/s00284-022-02807-7. Curr Microbiol. 2022. PMID: 35292865 Free PMC article. Review.
-
Vimentin Intermediate Filaments Mediate Cell Morphology on Viscoelastic Substrates.ACS Appl Bio Mater. 2022 Feb 21;5(2):552-561. doi: 10.1021/acsabm.1c01046. Epub 2022 Jan 7. ACS Appl Bio Mater. 2022. PMID: 34995457 Free PMC article.
-
Structure and function of vimentin in the generation and secretion of extracellular vimentin in response to inflammation.Cell Commun Signal. 2025 Apr 18;23(1):187. doi: 10.1186/s12964-025-02194-z. Cell Commun Signal. 2025. PMID: 40251523 Free PMC article. Review.
-
Recombinant human plasma gelsolin reverses increased permeability of the blood-brain barrier induced by the spike protein of the SARS-CoV-2 virus.J Neuroinflammation. 2022 Nov 24;19(1):282. doi: 10.1186/s12974-022-02642-4. J Neuroinflammation. 2022. PMID: 36434734 Free PMC article.
References
-
- Aguiar JA; Tremblay BJ-M; Mansfield MJ; Woody O; Lobb B; Banerjee A; Chandiramohan A; Tiessen N; Cao Q; Dvorkin-Gheva A; Revill S; Miller MS; Carlsten C; Organ L; Joseph C; John A; Hanson P; Austin RC; McManus BM; Jenkins G; Mossman K; Ask K; Doxey AC; Hirota JA, Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. European Respiratory Journal 2020, 56 (3), 2001123. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

