Transmembrane Protein TMEM230, a Target of Glioblastoma Therapy
- PMID: 34867197
- PMCID: PMC8636015
- DOI: 10.3389/fncel.2021.703431
Transmembrane Protein TMEM230, a Target of Glioblastoma Therapy
Abstract
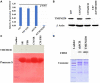
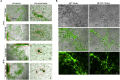
Glioblastomas (GBM) are the most aggressive tumors originating in the brain. Histopathologic features include circuitous, disorganized, and highly permeable blood vessels with intermittent blood flow. These features contribute to the inability to direct therapeutic agents to tumor cells. Known targets for anti-angiogenic therapies provide minimal or no effect in overall survival of 12-15 months following diagnosis. Identification of novel targets therefore remains an important goal for effective treatment of highly vascularized tumors such as GBM. We previously demonstrated in zebrafish that a balanced level of expression of the transmembrane protein TMEM230/C20ORF30 was required to maintain normal blood vessel structural integrity and promote proper vessel network formation. To investigate whether TMEM230 has a role in the pathogenesis of GBM, we analyzed its prognostic value in patient tumor gene expression datasets and performed cell functional analysis. TMEM230 was found necessary for growth of U87-MG cells, a model of human GBM. Downregulation of TMEM230 resulted in loss of U87 migration, substratum adhesion, and re-passaging capacity. Conditioned media from U87 expressing endogenous TMEM230 induced sprouting and tubule-like structure formation of HUVECs. Moreover, TMEM230 promoted vascular mimicry-like behavior of U87 cells. Gene expression analysis of 702 patients identified that TMEM230 expression levels distinguished high from low grade gliomas. Transcriptomic analysis of patients with gliomas revealed molecular pathways consistent with properties observed in U87 cell assays. Within low grade gliomas, elevated TMEM230 expression levels correlated with reduced overall survival independent from tumor subtype. Highest level of TMEM230 correlated with glioblastoma and ATP-dependent microtubule kinesin motor activity, providing a direction for future therapeutic intervention. Our studies support that TMEM230 has both glial tumor and endothelial cell intracellular and extracellular functions. Elevated levels of TMEM230 promote glial tumor cell migration, extracellular scaffold remodeling, and hypervascularization and abnormal formation of blood vessels. Downregulation of TMEM230 expression may inhibit both low grade glioma and glioblastoma tumor progression and promote normalization of abnormally formed blood vessels. TMEM230 therefore is both a promising anticancer and antiangiogenic therapeutic target for inhibiting GBM tumor cells and tumor-driven angiogenesis.
Keywords: angiogenesis and normalization of vascular network; anticancer and antiangiogenic therapy; cargo vesicle transport; glioma; kinesin motor proteins; tumor cell migration and adhesion.
Copyright © 2021 Cocola, Magnaghi, Abeni, Pelucchi, Martino, Vilardo, Piscitelli, Consiglio, Grillo, Mosca, Gualtierotti, Mazzaccaro, La Sala, Di Pietro, Palizban, Liuni, DePedro, Morara, Nano, Kehler, Greve, Noghero, Marazziti, Bussolino, Bellipanni, D’Agnano, Götte, Zucchi and Reinbold.
Conflict of interest statement
IZ and RR have a patent accepted concerning the use of Agents that modulate TMEM230 in tumor associated angiogenesis. Patent Application International Publication number: 20200247882. Agents that modulate TMEM230 as angiogenesis regulators and that detect TMEM230 AS markers of metastasis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aldape K., Zadeh G., Mansouri S., Reifenberger G., Von Deimling A. (2015). Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 129 829–848. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

