Magnetic Resonance Imaging Sequence Identification Using a Metadata Learning Approach
- PMID: 34867254
- PMCID: PMC8635782
- DOI: 10.3389/fninf.2021.622951
Magnetic Resonance Imaging Sequence Identification Using a Metadata Learning Approach
Abstract
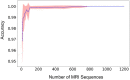
Despite the wide application of the magnetic resonance imaging (MRI) technique, there are no widely used standards on naming and describing MRI sequences. The absence of consistent naming conventions presents a major challenge in automating image processing since most MRI software require a priori knowledge of the type of the MRI sequences to be processed. This issue becomes increasingly critical with the current efforts toward open-sharing of MRI data in the neuroscience community. This manuscript reports an MRI sequence detection method using imaging metadata and a supervised machine learning technique. Three datasets from the Brain Center for Ontario Data Exploration (Brain-CODE) data platform, each involving MRI data from multiple research institutes, are used to build and test our model. The preliminary results show that a random forest model can be trained to accurately identify MRI sequence types, and to recognize MRI scans that do not belong to any of the known sequence types. Therefore the proposed approach can be used to automate processing of MRI data that involves a large number of variations in sequence names, and to help standardize sequence naming in ongoing data collections. This study highlights the potential of the machine learning approaches in helping manage health data.
Keywords: AI-assisted data management; MRI sequence naming standardization; data share and exchange; health data; machine learning; metadata learning.
Copyright © 2021 Liang, Beaton, Arnott, Gee, Zamyadi, Bartha, Symons, MacQueen, Hassel, Lerch, Anagnostou, Lam, Frey, Milev, Müller, Kennedy, Scott, The ONDRI Investigators and Strother.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abbasi S., Tajeripour F. (2017). Detection of brain tumor in 3D MRI images using local binary patterns and histogram orientation gradient. Neurocomputing 219 526–535. 10.1016/j.neucom.2016.09.051 - DOI
-
- Beaton D., Adni A., Saporta G., Abdi H. (2019). A generalization of partial least squares regression and correspondence analysis for categorical and mixed data: An application with the ADNI data. bioRxiv 2019:598888. 10.1101/598888 - DOI
-
- Breiman L. (2001). Random Forests. Machine Learn. 45 5–32.
LinkOut - more resources
Full Text Sources
Other Literature Sources

