Knockout of AMPKα2 Blocked the Protection of Sestrin2 Overexpression Against Cardiac Hypertrophy Induced by Pressure Overload
- PMID: 34867324
- PMCID: PMC8635785
- DOI: 10.3389/fphar.2021.716884
Knockout of AMPKα2 Blocked the Protection of Sestrin2 Overexpression Against Cardiac Hypertrophy Induced by Pressure Overload
Abstract
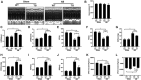
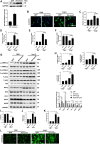
Objectives: Sestrin2 (Sesn2) has been demonstrated to be a cysteine sulfinyl reductase and protects cells from multiple stress insults, including hypoxia, endoplasmic reticulum stress, and oxidative stress. However, the roles and mechanisms of Sesn2 in pressure overload-induced mouse cardiac hypertrophy have not been clearly clarified. This study intended to investigate whether sestrin2 (Sesn2) overexpression could prevent pressure overload-induced cardiac hypertrophy via an AMPKα2 dependent pathway through conditional knockout of AMPKα2. Methods and results: Sesn2 expression was significantly increased in mice hearts at 2 and 4 weeks after aortic banding (AB) surgery, but decreased to 60-70% of the baseline at 8 weeks. Sesn2 overexpression (at 3, 6, and 9 folds) showed little cardiac genetic toxicity in transgenic mice. Cardiac dysfunctions induced by pressure overload were attenuated by cardiomyocyte-specific Sesn2 overexpression when measured by echocardiography and hemodynamic analysis. Results of HE and PSR staining showed that Sesn2 overexpression significantly alleviated cardiac hypertrophy and fibrosis in mice hearts induced by pressure overload. Meanwhile, adenovirus-mediated-Sesn2 overexpression markedly suppressed angiotensin II-induced neonatal rat cardiomyocyte hypertrophy in vitro. Mechanistically, Sesn2 overexpression increased AMPKα2 phosphorylation but inhibited mTORC1 phosphorylation. The cardiac protections of Sesn2 overexpression were also via regulating oxidative stress by enhancing Nrf2/HO-1 signaling, restoring SOD activity, and suppressing NADPH activity. Particularly, we first proved the vital role of AMPKα2 in the regulation of Sesn2 with AMPKα2 knockout (AMPKα2-/-) mice and Sesn2 transgenic mice crossed with AMPKα2-/-, since Sesn2 overexpression failed to improve cardiac function, inhibit cardiac hypertrophy and fibrosis, and attenuate oxidative stress after AMPKα2 knockout. Conclusion: This study uniquely revealed that Sesn2 overexpression showed little genetic toxicity in mice hearts and inhibited mTORC1 activation and oxidative stress to protect against pressure overload-induced cardiac hypertrophy in an AMPKα2 dependent pathway. Thus, interventions through promoting Sesn2 expression might be a potential strategy for treating pathological cardiac hypertrophy and heart failure.
Keywords: AMPKα; Sestrin2; cardiac hypertrophy; fibrosis; oxidative stress.
Copyright © 2021 Zhang, Liao, Feng, Mou, Li, Aiyasiding, Lin, Ding, Zhou, Yan, Chen and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Substrate metabolism regulated by Sestrin2-mTORC1 alleviates pressure overload-induced cardiac hypertrophy in aged heart.Redox Biol. 2020 Sep;36:101637. doi: 10.1016/j.redox.2020.101637. Epub 2020 Jul 9. Redox Biol. 2020. PMID: 32863202 Free PMC article.
-
Liquiritin Attenuates Pathological Cardiac Hypertrophy by Activating the PKA/LKB1/AMPK Pathway.Front Pharmacol. 2022 May 3;13:870699. doi: 10.3389/fphar.2022.870699. eCollection 2022. Front Pharmacol. 2022. PMID: 35592411 Free PMC article.
-
Peroxiredoxin-1 ameliorates pressure overload-induced cardiac hypertrophy and fibrosis.Biomed Pharmacother. 2020 Sep;129:110357. doi: 10.1016/j.biopha.2020.110357. Epub 2020 Jun 9. Biomed Pharmacother. 2020. PMID: 32531679
-
Sestrin2: multifaceted functions, molecular basis, and its implications in liver diseases.Cell Death Dis. 2023 Feb 25;14(2):160. doi: 10.1038/s41419-023-05669-4. Cell Death Dis. 2023. PMID: 36841824 Free PMC article. Review.
-
Sestrin2 serves as a scaffold protein to maintain cardiac energy and metabolic homeostasis during pathological stress.FASEB J. 2024 Oct 31;38(20):e70106. doi: 10.1096/fj.202401404R. FASEB J. 2024. PMID: 39404019 Free PMC article. Review.
Cited by
-
Sestrin2 in diabetes and diabetic complications.Front Endocrinol (Lausanne). 2023 Oct 18;14:1274686. doi: 10.3389/fendo.2023.1274686. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37920252 Free PMC article. Review.
-
Muscular Sestrins: Roles in Exercise Physiology and Stress Resistance.Biomolecules. 2023 Apr 23;13(5):722. doi: 10.3390/biom13050722. Biomolecules. 2023. PMID: 37238592 Free PMC article. Review.
-
Oxidative Stress-Induced Protein of SESTRIN2 in Cardioprotection Effect.Dis Markers. 2022 Jul 29;2022:7439878. doi: 10.1155/2022/7439878. eCollection 2022. Dis Markers. 2022. PMID: 35937943 Free PMC article. Review.
-
Sestrin2 can alleviate endoplasmic reticulum stress to improve traumatic brain injury by activating AMPK/mTORC1 signaling pathway.Metab Brain Dis. 2024 Mar;39(3):439-452. doi: 10.1007/s11011-023-01323-2. Epub 2023 Dec 4. Metab Brain Dis. 2024. PMID: 38047978 Review.
-
Targeting on Nrf2/Sesn2 Signaling to Rescue Cardiac Dysfunction during High-Fat Diet-Induced Obesity.Cells. 2022 Aug 22;11(16):2614. doi: 10.3390/cells11162614. Cells. 2022. PMID: 36010689 Free PMC article.
References
LinkOut - more resources
Full Text Sources

