Granulocyte Colony-Stimulating Factor Treatment Before Radiotherapy Protects Against Radiation-Induced Liver Disease in Mice
- PMID: 34867327
- PMCID: PMC8634713
- DOI: 10.3389/fphar.2021.725084
Granulocyte Colony-Stimulating Factor Treatment Before Radiotherapy Protects Against Radiation-Induced Liver Disease in Mice
Abstract
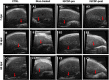
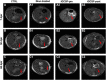
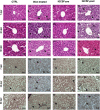
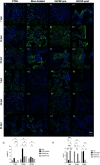
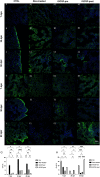
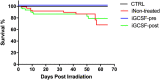
Radiation-induced liver disease (RILD) remains a major problem resulting from radiotherapy. In this scenario, immunotherapy with granulocyte colony-stimulating factor (G-CSF) arises as an attractive approach that might improve the injured liver. Here, we investigated G-CSF administration's impact before and after liver irradiation exposure using an association of alcohol consumption and local irradiation to induce liver disease model in C57BL/6 mice. Male and female mice were submitted to a previous alcohol-induced liver injury protocol with water containing 5% alcohol for 90 days. Then, the animals were treated with G-CSF (100 μg/kg/d) for 3 days before or after liver irradiation (18 Gy). At days 7, 30, and 60 post-radiation, non-invasive liver images were acquired by ultrasonography, magnetic resonance, and computed tomography. Biochemical and histological evaluations were performed to verify whether G-CSF could prevent liver tissue damage or reverse the acute liver injury. Our data showed that the treatment with G-CSF before irradiation effectively improved morphofunctional parameters caused by RILD, restoring histological arrangement, promoting liver regeneration, preserving normal organelles distribution, and glycogen granules. The amount of OV-6 and F4/80-positive cells increased, and α-SMA positive cells' presence was normalized. Additionally, prior G-CSF administration preserved serum biochemical parameters and increased the survival rates (100%). On the other hand, after irradiation, the treatment showed a slight improvement in survival rates (79%) and did not ameliorate RILD. Overall, our data suggest that G-CSF administration before radiation might be an immunotherapeutic alternative to radiotherapy planning to avoid RILD.
Keywords: G-CSF; alcohol; computed tomography; immunotherapy; irradiation; liver; magnetic resonance imaging; ultrasonography.
Copyright © 2021 Ramos, Dias, Nunes De Moraes, Meireles Ferreira, Souza, Gutfilen, Barboza, Ferreira Pimentel, Paz Batista, Kasai-Brunswick, Fortes, De Andrade and Goldenberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
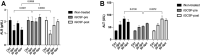



Similar articles
-
Administration of granulocyte colony-stimulating factor ameliorates radiation-induced hepatic fibrosis in mice.Transplant Proc. 2010 Nov;42(9):3833-9. doi: 10.1016/j.transproceed.2010.09.010. Transplant Proc. 2010. PMID: 21094866
-
Effect of human recombinant granulocyte colony-stimulating factor on induction of myeloid leukemias by X-irradiation in mice.Blood. 1993 Oct 1;82(7):2163-8. Blood. 1993. PMID: 7691250
-
Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation.Cytokine. 2012 Jun;58(3):406-14. doi: 10.1016/j.cyto.2012.03.011. Epub 2012 Apr 8. Cytokine. 2012. PMID: 22487481
-
Partial irradiation of the liver.Semin Radiat Oncol. 2001 Jul;11(3):240-6. doi: 10.1053/srao.2001.23485. Semin Radiat Oncol. 2001. PMID: 11447581 Review.
-
[Colony-stimulating factors in cancer chemo- and radiotherapy].Gan To Kagaku Ryoho. 1988 Apr;15(4 Pt 2-1):810-9. Gan To Kagaku Ryoho. 1988. PMID: 2455481 Review. Japanese.
Cited by
-
Transcriptomic analysis of BM-MSCs identified EGR1 as a transcription factor to fully exploit their therapeutic potential.Biochim Biophys Acta Mol Cell Res. 2024 Dec;1871(8):119818. doi: 10.1016/j.bbamcr.2024.119818. Epub 2024 Aug 19. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 39168411 Free PMC article.
-
Liver regeneration as treatment target for severe alcoholic hepatitis.World J Gastroenterol. 2022 Aug 28;28(32):4557-4573. doi: 10.3748/wjg.v28.i32.4557. World J Gastroenterol. 2022. PMID: 36157937 Free PMC article. Review.
References
-
- Carvalho A. B., Quintanilha L. F., Dias J. V., Paredes B. D., Mannheimer E. G., Carvalho F. G., et al. (2008). Bone Marrow Multipotent Mesenchymal Stromal Cells Do Not Reduce Fibrosis or Improve Function in a Rat Model of Severe Chronic Liver Injury. Stem Cells 26 (5), 1307–1314. 10.1634/stemcells.2007-0941 - DOI - PubMed
LinkOut - more resources
Full Text Sources

