Analysis of Cardiac Vibration Signals Acquired From a Novel Implant Placed on the Gastric Fundus
- PMID: 34867453
- PMCID: PMC8640497
- DOI: 10.3389/fphys.2021.748367
Analysis of Cardiac Vibration Signals Acquired From a Novel Implant Placed on the Gastric Fundus
Abstract
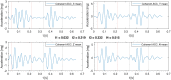
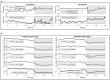
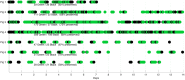
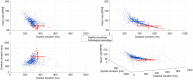
The analysis of cardiac vibration signals has been shown as an interesting tool for the follow-up of chronic pathologies involving the cardiovascular system, such as heart failure (HF). However, methods to obtain high-quality, real-world and longitudinal data, that do not require the involvement of the patient to correctly and regularly acquire these signals, remain to be developed. Implantable systems may be a solution to this observability challenge. In this paper, we evaluate the feasibility of acquiring useful electrocardiographic (ECG) and accelerometry (ACC) data from an innovative implant located in the gastric fundus. In a first phase, we compare data acquired from the gastric fundus with gold standard data acquired from surface sensors on 2 pigs. A second phase investigates the feasibility of deriving useful hemodynamic markers from these gastric signals using data from 4 healthy pigs and 3 pigs with induced HF with longitudinal recordings. The following data processing chain was applied to the recordings: (1) ECG and ACC data denoising, (2) noise-robust real-time QRS detection from ECG signals and cardiac cycle segmentation, (3) Correlation analysis of the cardiac cycles and computation of coherent mean from aligned ECG and ACC, (4) cardiac vibration components segmentation (S1 and S2) from the coherent mean ACC data, and (5) estimation of signal context and a signal-to-noise ratio (SNR) on both signals. Results show a high correlation between the markers acquired from the gastric and thoracic sites, as well as pre-clinical evidence on the feasibility of chronic cardiovascular monitoring from an implantable cardiac device located at the gastric fundus, the main challenge remains on the optimization of the signal-to-noise ratio, in particular for the handling of some sources of noise that are specific to the gastric acquisition site.
Keywords: biomedical signal processing; cardiac vibration signals; heart failure; implantable devices; seismocardiogram (SCG).
Copyright © 2021 Areiza-Laverde, Dopierala, Senhadji, Boucher, Gumery and Hernández.
Conflict of interest statement
CD is employed by SentinHealth SA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Bordachar P., Labrousse L., Ploux S., Thambo J.-B., Lafitte S., Reant P., et al. . (2008). Validation of a new noninvasive device for the monitoring of peak endocardial acceleration in pigs: implications for optimization of pacing site and configuration. J. Cardiovasc. Electrophysiol. 19, 725–729. 10.1111/j.1540-8167.2008.01105.x - DOI - PubMed
-
- Calvo M., Bonnet J.-L., Le Rolle V., Lemonnier M., Yasuda S., Oosterlinck W., et al. . (2018). Evaluation of three-dimensional accelerometers for the study of left ventricular contractility, in 2018 Computing in Cardiology Conference (CinC), Vol. 45, (Maastricht: IEEE; ), 1–4.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

