Lactobacillus plantarum Lipoteichoic Acids Possess Strain-Specific Regulatory Effects on the Biofilm Formation of Dental Pathogenic Bacteria
- PMID: 34867884
- PMCID: PMC8636137
- DOI: 10.3389/fmicb.2021.758161
Lactobacillus plantarum Lipoteichoic Acids Possess Strain-Specific Regulatory Effects on the Biofilm Formation of Dental Pathogenic Bacteria
Abstract
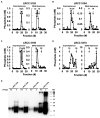
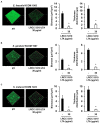
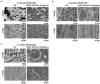
Bacterial biofilm residing in the oral cavity is closely related to the initiation and persistence of various dental diseases. Previously, we reported the anti-biofilm activity of Lactobacillus plantarum lipoteichoic acid (Lp.LTA) on a representative dental cariogenic pathogen, Streptococcus mutans. Since LTA structure varies in a bacterial strain-specific manner, LTAs from various L. plantarum strains may have differential anti-biofilm activity due to their distinct molecular structures. In the present study, we isolated Lp.LTAs from four different strains of L. plantarum (LRCC 5193, 5194, 5195, and 5310) and compared their anti-biofilm effects on the dental pathogens, including S. mutans, Enterococcus faecalis, and Streptococcus gordonii. All Lp.LTAs similarly inhibited E. faecalis biofilm formation in a dose-dependent manner. However, their effects on S. gordonii and S. mutans biofilm formation were different: LRCC 5310 Lp.LTA most effectively suppressed the biofilm formation of all strains of dental pathogens, while Lp.LTAs from LRCC 5193 and 5194 hardly inhibited or even enhanced the biofilm formation. Furthermore, LRCC 5310 Lp.LTA dramatically reduced the biofilm formation of the dental pathogens on the human dentin slice infection model. Collectively, these results suggest that Lp.LTAs have strain-specific regulatory effects on biofilm formation of dental pathogens and LRCC 5310 Lp.LTA can be used as an effective anti-biofilm agent for the prevention of dental infectious diseases.
Keywords: Lactobacillus plantarum; biofilm; dental pathogens; human dentin slice; lipoteichoic acid.
Copyright © 2021 Lee, Im, Park, Jeong, Park, Yoon, Park and Han.
Conflict of interest statement
MP, SY, and JP are employed by Lotte R&D Center. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Castillo Pedraza M. C., Novais T. F., Faustoferri R. C., Quivey R. G., Jr., Terekhov A., Hamaker B. R., et al. . (2017). Extracellular DNA and lipoteichoic acids interact with exopolysaccharides in the extracellular matrix of Streptococcus mutans biofilms. Biofouling 33, 722–740. doi: 10.1080/08927014.2017.1361412, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

