Therapeutic Potential of a Novel Bifidobacterium Identified Through Microbiome Profiling of RA Patients With Different RF Levels
- PMID: 34867956
- PMCID: PMC8634832
- DOI: 10.3389/fimmu.2021.736196
Therapeutic Potential of a Novel Bifidobacterium Identified Through Microbiome Profiling of RA Patients With Different RF Levels
Abstract
The potential therapeutic effects of probiotic bacteria in rheumatoid arthritis (RA) remain controversial. Thus, this study aimed to discover potential therapeutic bacteria based on the relationship between the gut microbiome and rheumatoid factor (RF) in RA. Bacterial genomic DNA was extracted from the fecal samples of 93 RA patients and 16 healthy subjects. Microbiota profiling was conducted through 16S rRNA sequencing and bioinformatics analyses. The effects of Bifidobacterium strains on human peripheral blood mononuclear cells and collagen-induced arthritis (CIA) mice were assessed. Significant differences in gut microbiota composition were observed in patients with different RF levels. The relative abundance of Bifidobacterium and Collinsella was lower in RF-high than in RF-low and RF-negative RA patients, while the relative abundance of Clostridium of Ruminococcaceae family was higher in RF-high than in RF-low and RF-negative patients. Among 10 differentially abundant Bifidobacterium, B. longum RAPO exhibited the strongest ability to inhibit IL-17 secretion. Oral administration of B. longum RAPO in CIA mice, obese CIA, and humanized avatar model significantly reduced RA incidence, arthritis score, inflammation, bone damage, cartilage damage, Th17 cells, and inflammatory cytokine secretion. Additionally, B. longum RAPO significantly inhibited Th17 cells and Th17-related genes-IL-17A, IRF4, RORC, IL-21, and IL-23R-in the PBMCs of rheumatoid arthritis patients. Our findings suggest that B. longum RAPO may alleviate RA by inhibiting the production of IL-17 and other proinflammatory mediators. The safety and efficacy of B. longum RAPO in patients with RA and other autoimmune disorders merit further investigation.
Keywords: Bifidobacterium longum; T helper 17 cell; microbiome; rheumatoid arthritis; rheumatoid factor.
Copyright © 2021 Jeong, Jhun, Lee, Na, Choi, Cho, Lee, Lee, Park, You, Kim, Park, Kwon, Cho, Ji and Park.
Conflict of interest statement
Authors YJ, S-JP, MP, BK and GJ was employed by company BIFIDO Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

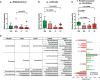



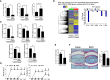
References
-
- Elagib KE, Borretzen M, Jonsson R, Haga HJ, Thoen J, Thompson KM, et al. . Rheumatoid Factors in Primary Sjogren’s Syndrome (pSS) Use Diverse VH Region Genes, the Majority of Which Show No Evidence of Somatic Hypermutation. Clin Exp Immunol (1999) 117(2):388–94. doi: 10.1046/j.1365-2249.1999.00963.x - DOI - PMC - PubMed
-
- Hayahara C, Ikeda K, Sakanishi Y, Ota T. Incidence of Serum Rheumatoid Factors in Elder non-Rheumatic Individuals. Rinsho Byori (2010) 58(3):211–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

