Anti-Drug Antibodies in Pigtailed Macaques Receiving HIV Broadly Neutralising Antibody PGT121
- PMID: 34867979
- PMCID: PMC8636046
- DOI: 10.3389/fimmu.2021.749891
Anti-Drug Antibodies in Pigtailed Macaques Receiving HIV Broadly Neutralising Antibody PGT121
Abstract
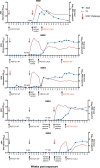
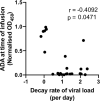
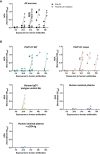
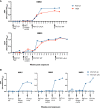
Broadly neutralising antibodies (bNAbs) may play an important role in future strategies for HIV control. The development of anti-drug antibody (ADA) responses can reduce the efficacy of passively transferred bNAbs but the impact of ADA is imperfectly understood. We previously showed that therapeutic administration of the anti-HIV bNAb PGT121 (either WT or LALA version) controlled viraemia in pigtailed macaques with ongoing SHIV infection. We now report on 23 macaques that had multiple treatments with PGT121. We found that an increasing number of intravenous doses of PGT121 or human IgG1 isotype control antibodies (2-4 doses) results in anti-PGT121 ADA induction and low plasma concentrations of PGT121. ADA was associated with poor or absent suppression of SHIV viremia. Notably, ADA within macaque plasma recognised another human bNAb 10E8 but did not bind to the variable domains of PGT121, suggesting that ADA were primarily directed against the constant regions of the human antibodies. These findings have implications for the development of preclinical studies examining multiple infusions of human bNAbs.
Keywords: HIV; PGT121; anti-drug antibodies (ADA); broadly neutralizing antibodies (bNAb); pigtailed macaque.
Copyright © 2021 Lee, Reynaldi, Amarasena, Davenport, Parsons and Kent.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, Saunders JG, et al. Safety and Pharmacokinetics of the Fc-Modified HIV-1 Human Monoclonal Antibody VRC01LS: A Phase 1 Open-Label Clinical Trial in Healthy Adults. PloS Med (2018) 15(1):e1002493. doi: 10.1371/journal.pmed.1002493 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

