A New Murine Model of Primary Autoimmune Hemolytic Anemia (AIHA)
- PMID: 34867985
- PMCID: PMC8634489
- DOI: 10.3389/fimmu.2021.752330
A New Murine Model of Primary Autoimmune Hemolytic Anemia (AIHA)
Abstract
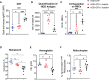
Loss of humoral tolerance to red blood cells (RBCs) can lead to autoimmune hemolytic anemia (AIHA), a severe, and sometimes fatal disease. Patients with AIHA present with pallor, fatigue, decreased hematocrit, and splenomegaly. While secondary AIHA is associated with lymphoproliferative disorders, infections, and more recently, as an adverse event secondary to cancer immunotherapy, the etiology of primary AIHA is unknown. Several therapeutic strategies are available; however, there are currently no licensed treatments for AIHA and few therapeutics offer treatment-free durable remission. Moreover, supportive care with RBC transfusions can be challenging as most autoantibodies are directed against ubiquitous RBC antigens; thus, virtually all RBC donor units are incompatible. Given the severity of AIHA and the lack of treatment options, understanding the cellular and molecular mechanisms that facilitate the breakdown in tolerance would provide insight into new therapeutics. Herein, we report a new murine model of primary AIHA that reflects the biology observed in patients with primary AIHA. Production of anti-erythrocyte autoantibodies correlated with sex and age, and led to RBC antigen modulation, complement fixation, and anemia, as determined by decreased hematocrit and hemoglobin values and increased reticulocytes in peripheral blood. Moreover, autoantibody-producing animals developed splenomegaly, with altered splenic architecture characterized by expanded white pulp areas and nearly diminished red pulp areas. Additional analysis suggested that compensatory extramedullary erythropoiesis occurred as there were increased frequencies of RBC progenitors detectable in the spleen. No significant correlations between AIHA onset and inflammatory status or microbiome were observed. To our knowledge, this is the first report of a murine model that replicates observations made in humans with idiopathic AIHA. Thus, this is a tractable murine model of AIHA that can serve as a platform to identify key cellular and molecular pathways that are compromised, thereby leading to autoantibody formation, as well as testing new therapeutics and management strategies.
Keywords: RBC (red-blood-cell); autoimmune disease; autoimmune hemolytic anemia; erythrocyte; idiopathic autoimmune hemolytic anemia; primary autoimmune hemolytic anemia; red blood cell; tolerance.
Copyright © 2021 Dei Zotti, Qiu, La Carpia, Moriconi and Hudson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

