Impairment of Multiple Mitochondrial Energy Metabolism Pathways in the Heart of Chagas Disease Cardiomyopathy Patients
- PMID: 34867990
- PMCID: PMC8633876
- DOI: 10.3389/fimmu.2021.755782
Impairment of Multiple Mitochondrial Energy Metabolism Pathways in the Heart of Chagas Disease Cardiomyopathy Patients
Abstract
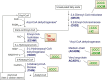
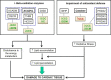
Chagas disease cardiomyopathy (CCC) is an inflammatory dilated cardiomyopathy occurring in 30% of the 6 million infected with the protozoan Trypanosoma cruzi in Latin America. Survival is significantly lower in CCC than ischemic (IC) and idiopathic dilated cardiomyopathy (DCM). Previous studies disclosed a selective decrease in mitochondrial ATP synthase alpha expression and creatine kinase activity in CCC myocardium as compared to IDC and IC, as well as decreased in vivo myocardial ATP production. Aiming to identify additional constraints in energy metabolism specific to CCC, we performed a proteomic study in myocardial tissue samples from CCC, IC and DCM obtained at transplantation, in comparison with control myocardial tissue samples from organ donors. Left ventricle free wall myocardial samples were subject to two-dimensional electrophoresis with fluorescent labeling (2D-DIGE) and protein identification by mass spectrometry. We found altered expression of proteins related to mitochondrial energy metabolism, cardiac remodeling, and oxidative stress in the 3 patient groups. Pathways analysis of proteins differentially expressed in CCC disclosed mitochondrial dysfunction, fatty acid metabolism and transmembrane potential of mitochondria. CCC patients' myocardium displayed reduced expression of 22 mitochondrial proteins belonging to energy metabolism pathways, as compared to 17 in DCM and 3 in IC. Significantly, 6 beta-oxidation enzymes were reduced in CCC, while only 2 of them were down-regulated in DCM and 1 in IC. We also observed that the cytokine IFN-gamma, previously described with increased levels in CCC, reduces mitochondrial membrane potential in cardiomyocytes. Results suggest a major reduction of mitochondrial energy metabolism and mitochondrial dysfunction in CCC myocardium which may be in part linked to IFN-gamma. This may partially explain the worse prognosis of CCC as compared to DCM or IC.
Keywords: chronic Chagas disease cardiomyopathy; energy metabolism; idiopathic dilated cardiomyopathy; interferon-gamma; ischemic cardiomyopathy; mitochondria; proteomics; two-dimensional electrophoresis with fluorescent labeling.
Copyright © 2021 Teixeira, Ducret, Langen, Nogoceke, Santos, Silva Nunes, Benvenuti, Levy, Bydlowski, Bocchi, Kuramoto Takara, Fiorelli, Stolf, Pomeranzeff, Chevillard, Kalil and Cunha-Neto.
Conflict of interest statement
PT, AD, and EN are current employees of F. Hoffmann-La Roche Ltd and may own company stock. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Libby P, Braunwald E. Braunwald’s Heart Disease : A Textbook of Cardiovascular Medicine. Philadelphia: Saunders/Elsevier; (2008).
-
- Bocchi EA. Update on Indications and Results of the Surgical Treatment of Heart Failure. Arq Bras Cardiol (1994) 63(6):523–30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

