Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 mRNA Vaccine in Older People
- PMID: 34868051
- PMCID: PMC8637126
- DOI: 10.3389/fimmu.2021.778679
Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 mRNA Vaccine in Older People
Abstract
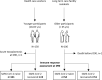
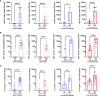
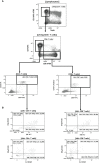
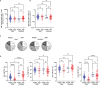
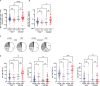
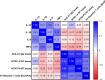
Long-term care facility (LTCF) older residents display physiological alterations of cellular and humoral immunity that affect vaccine responses. Preliminary reports suggested a low early postvaccination antibody response against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The aim of this study was to focus on the specific T-cell response. We quantified S1-specific IgG, neutralizing antibody titers, total specific IFNγ-secreting T cells by ELISpot, and functionality of CD4+- and CD8+-specific T cells by flow cytometry, after two doses of the BNT162b2 vaccine in younger and older people, with and without previous COVID-19 infection (hereafter referred to as COVID-19-recovered and COVID-19-naive subjects, respectively). Frailty, nutritional, and immunosenescence parameters were collected at baseline in COVID-19-naive older people. We analyzed the immune response in 129 young adults (median age 44.0 years) and 105 older residents living in a LCTF (median age 86.5 years), 3 months after the first injection. Humoral and cellular memory responses were dramatically impaired in the COVID-19-naive older (n = 54) compared with the COVID-19-naive younger adults (n = 121). Notably, older participants' neutralizing antibodies were 10 times lower than the younger's antibody titers (p < 0.0001) and LCTF residents also had an impaired functional T-cell response: the frequencies of IFNγ+ and IFNγ+IL-2+TNFα+ cells among specific CD4+ T cells, and the frequency of specific CD8+ T cells were lower in COVID-19-naive older participants than in COVID-19-naive young adults (p < 0.0001 and p = 0.0018, respectively). However, COVID-19-recovered older participants (n = 51) had greater antibody and T-cell responses, including IFNγ+ and IFNγ+IL-2+TNFα+-specific CD4+ T cells (p < 0.0001), as well as TNFα+-specific CD8+ T cells (p < 0.001), than COVID-19-naive older adults. We also observed that "inflammageing" and particularly high plasma levels of TNFα was associated to poor antibody response in the older participants. In conclusion, our results show that the COVID-19-naive older people had low counts and impaired specific CD4+ and CD8+ T cells, in addition to impaired antibody response, and that specific studies are warranted to assess the efficiency of SARS-CoV-2 mRNA-based vaccines, as in other immunocompromised subjects. Our study also shows that, despite their physiological alterations of immunity, vaccination is highly efficient in boosting the prior natural memory response in COVID-19-recovered older people.
Keywords: SARS – CoV – 2; T cells response; mRNA vaccination; older people and ageing; vaccine.
Copyright © 2021 Demaret, Corroyer-Simovic, Alidjinou, Goffard, Trauet, Miczek, Vuotto, Dendooven, Huvent-Grelle, Podvin, Dreuil, Faure, Deplanque, Bocket, Duhamel, Labreuche, Sobaszek, Hisbergues, Puisieux, Labalette and Lefèvre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

