Structural Evolution of TIR-Domain Signalosomes
- PMID: 34868065
- PMCID: PMC8635717
- DOI: 10.3389/fimmu.2021.784484
Structural Evolution of TIR-Domain Signalosomes
Abstract
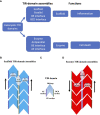
TIR (Toll/interleukin-1 receptor/resistance protein) domains are cytoplasmic domains widely found in animals and plants, where they are essential components of the innate immune system. A key feature of TIR-domain function in signaling is weak and transient self-association and association with other TIR domains. An additional new role of TIR domains as catalytic enzymes has been established with the recent discovery of NAD+-nucleosidase activity by several TIR domains, mostly involved in cell-death pathways. Although self-association of TIR domains is necessary in both cases, the functional specificity of TIR domains is related in part to the nature of the TIR : TIR interactions in the respective signalosomes. Here, we review the well-studied TIR domain-containing proteins involved in eukaryotic immunity, focusing on the structures, interactions and their corresponding functional roles. Structurally, the signalosomes fall into two separate groups, the scaffold and enzyme TIR-domain assemblies, both of which feature open-ended complexes with two strands of TIR domains, but differ in the orientation of the two strands. We compare and contrast how TIR domains assemble and signal through distinct scaffolding and enzymatic roles, ultimately leading to distinct cellular innate-immunity and cell-death outcomes.
Keywords: axon degeneration; cell-death signaling; innate immunity; plant disease resistance; protein structure; protein-protein interactions; signaling by cooperative assembly formation (SCAF); toll/interleukin-1 receptor/resistance protein.
Copyright © 2021 Nimma, Gu, Maruta, Li, Pan, Saikot, Lim, McGuinness, Zaoti, Li, Desa, Manik, Nanson and Kobe.
Conflict of interest statement
BK is a shareholder of Disarm Therapeutics, a wholly owned subsidiary of Eli Lilly & Company. BK is a consultant to Disarm Therapeutics. BK and WG receive research funding from Disarm Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
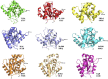


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

