Influenza Neuraminidase Characteristics and Potential as a Vaccine Target
- PMID: 34868073
- PMCID: PMC8635103
- DOI: 10.3389/fimmu.2021.786617
Influenza Neuraminidase Characteristics and Potential as a Vaccine Target
Abstract
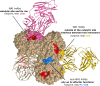
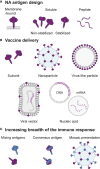
Neuraminidase of influenza A and B viruses plays a critical role in the virus life cycle and is an important target of the host immune system. Here, we highlight the current understanding of influenza neuraminidase structure, function, antigenicity, immunogenicity, and immune protective potential. Neuraminidase inhibiting antibodies have been recognized as correlates of protection against disease caused by natural or experimental influenza A virus infection in humans. In the past years, we have witnessed an increasing interest in the use of influenza neuraminidase to improve the protective potential of currently used influenza vaccines. A number of well-characterized influenza neuraminidase-specific monoclonal antibodies have been described recently, most of which can protect in experimental challenge models by inhibiting the neuraminidase activity or by Fc receptor-dependent mechanisms. The relative instability of the neuraminidase poses a challenge for protein-based antigen design. We critically review the different solutions that have been proposed to solve this problem, ranging from the inclusion of stabilizing heterologous tetramerizing zippers to the introduction of inter-protomer stabilizing mutations. Computationally engineered neuraminidase antigens have been generated that offer broad, within subtype protection in animal challenge models. We also provide an overview of modern vaccine technology platforms that are compatible with the induction of robust neuraminidase-specific immune responses. In the near future, we will likely see the implementation of influenza vaccines that confront the influenza virus with a double punch: targeting both the hemagglutinin and the neuraminidase.
Keywords: antigenic drift; correlate of protection; influenza; monoclonal antibodies; neuraminidase; vaccines.
Copyright © 2021 Creytens, Pascha, Ballegeer, Saelens and de Haan.
Conflict of interest statement
XS declares to receive funding from Sanofi Pasteur for research related to influenza vaccine development. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Paget J, Marquet R, Meijer A, van der Velden K. Influenza Activity in Europe During Eight Seasons (1999-2007): An Evaluation of the Indicators Used to Measure Activity and an Assessment of the Timing, Length and Course of Peak Activity (Spread) Across Europe. BMC Infect Dis (2007) 7(1):1–7. doi: 10.1186/1471-2334-7-141 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

