Safety and Immunogenicity Analysis of a Newcastle Disease Virus (NDV-HXP-S) Expressing the Spike Protein of SARS-CoV-2 in Sprague Dawley Rats
- PMID: 34868082
- PMCID: PMC8637447
- DOI: 10.3389/fimmu.2021.791764
Safety and Immunogenicity Analysis of a Newcastle Disease Virus (NDV-HXP-S) Expressing the Spike Protein of SARS-CoV-2 in Sprague Dawley Rats
Abstract
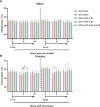
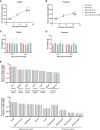
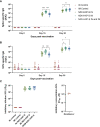
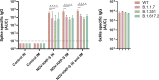
Despite global vaccination efforts, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to evolve and spread globally. Relatively high vaccination rates have been achieved in most regions of the United States and several countries worldwide. However, access to vaccines in low- and mid-income countries (LMICs) is still suboptimal. Second generation vaccines that are universally affordable and induce systemic and mucosal immunity are needed. Here we performed an extended safety and immunogenicity analysis of a second-generation SARS-CoV-2 vaccine consisting of a live Newcastle disease virus vector expressing a pre-fusion stabilized version of the spike protein (NDV-HXP-S) administered intranasally (IN), intramuscularly (IM), or IN followed by IM in Sprague Dawley rats. Local reactogenicity, systemic toxicity, and post-mortem histopathology were assessed after the vaccine administration, with no indication of severe local or systemic reactions. Immunogenicity studies showed that the three vaccination regimens tested elicited high antibody titers against the wild type SARS-CoV-2 spike protein and the NDV vector. Moreover, high antibody titers were induced against the spike of B.1.1.7 (alpha), B.1.351 (beta) and B.1.617.2 (delta) variants of concern (VOCs). Importantly, robust levels of serum antibodies with neutralizing activity against the authentic SARS-CoV-2 USA-WA1/2020 isolate were detected after the boost. Overall, our study expands the pre-clinical safety and immunogenicity characterization of NDV-HXP-S and reinforces previous findings in other animal models about its high immunogenicity. Clinical testing of this vaccination approach is ongoing in different countries including Thailand, Vietnam, Brazil and Mexico.
Keywords: COVID-19; SARS-CoV-2; immunogenicity; newcastle disease virus; rat model; safety; vaccine.
Copyright © 2021 Tcheou, Raskin, Singh, Kawabata, Bielak, Sun, González-Domínguez, Sather, García-Sastre, Palese, Krammer and Carreño.
Conflict of interest statement
The Icahn School of Medicine at Mount Sinai has filed patent applications relating to NDV-based SARS-CoV-2 vaccines which list WS, FK, AG-S and PP as co-inventors. FK is also listed as inventor on patent applications for SARS-CoV-2 serological assays filed by the Icahn School of Medicine at Mount Sinai. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. FK has consulted for Merck and Pfizer (before 2020) as well as Goldman Sachs, and is currently consulting for Pfizer, Seqirus and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. The Adolfo García-Sastre laboratory has received research support from Pfizer, Senhwa Biosciences, Kenall Manufacturing, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N-fold LLC, Model Medicines and Merck, outside of the reported work. AG-S has consulting agreements for the following companies involving cash and/or stock: Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Vaxalto, Pagoda, Accurius, Esperovax, Farmak, Applied Biological Laboratories, Pharmamar, and Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing SARS-CoV-2 spike: Results of a randomized vaccine-controlled phase I ADAPTCOV trial in Brazil.Vaccine. 2025 Apr 11;52:126680. doi: 10.1016/j.vaccine.2024.126680. Epub 2025 Mar 3. Vaccine. 2025. PMID: 40037239 Clinical Trial.
-
Intranasal SARS-CoV-2 Omicron variant vaccines elicit humoral and cellular mucosal immunity in female mice.EBioMedicine. 2024 Jul;105:105185. doi: 10.1016/j.ebiom.2024.105185. Epub 2024 Jun 7. EBioMedicine. 2024. PMID: 38848648 Free PMC article.
-
Safety and immunogenicity of an inactivated recombinant Newcastle disease virus vaccine expressing SARS-CoV-2 spike: A randomised, comparator-controlled, phase 2 trial.Vaccine. 2025 Jan 12;44:126542. doi: 10.1016/j.vaccine.2024.126542. Epub 2024 Nov 29. Vaccine. 2025. PMID: 39615342 Free PMC article. Clinical Trial.
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
-
SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants.Front Immunol. 2021 Jul 12;12:701501. doi: 10.3389/fimmu.2021.701501. eCollection 2021. Front Immunol. 2021. PMID: 34322129 Free PMC article. Review.
Cited by
-
Development of multidose thermotolerant formulations of a vector-based Covid-19 vaccine candidate, NDV-HXP-S in different product formats: Stability and preservative efficacy study.Vaccine X. 2024 Jul 27;20:100535. doi: 10.1016/j.jvacx.2024.100535. eCollection 2024 Oct. Vaccine X. 2024. PMID: 39189025 Free PMC article.
-
Evolving SARS-CoV-2 Vaccines: From Current Solutions to Broad-Spectrum Protection.Vaccines (Basel). 2025 Jun 12;13(6):635. doi: 10.3390/vaccines13060635. Vaccines (Basel). 2025. PMID: 40573967 Free PMC article. Review.
-
Development of SARS-CoV-2 animal vaccines using a stable and efficient NDV expression system.J Med Virol. 2023 Jan;95(1):e28237. doi: 10.1002/jmv.28237. Epub 2022 Oct 28. J Med Virol. 2023. PMID: 36258299 Free PMC article.
-
Negative-Strand RNA Virus-Vectored Vaccines.Methods Mol Biol. 2024;2786:51-87. doi: 10.1007/978-1-0716-3770-8_3. Methods Mol Biol. 2024. PMID: 38814390 Review.
-
Intranasal vaccination with an NDV-vectored SARS-CoV-2 vaccine protects against Delta and Omicron challenges.NPJ Vaccines. 2024 May 23;9(1):90. doi: 10.1038/s41541-024-00870-8. NPJ Vaccines. 2024. PMID: 38782986 Free PMC article.
References
-
- Centers for Disease Control and Prevention . How to Protect Yourself & Others (2021). Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/preventio....
-
- U.S. Food. Drug Administration . FDA Approves First COVID-19 Vaccine. (2021). Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-c....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

