A Comparison of Bevacizumab Plus TAS-102 and TAS-102 Monotherapy for Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis
- PMID: 34868908
- PMCID: PMC8637322
- DOI: 10.3389/fonc.2021.690515
A Comparison of Bevacizumab Plus TAS-102 and TAS-102 Monotherapy for Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis
Abstract
Backgrounds: As a new oral chemotherapy drug, TAS-102 is currently recommended as the third-line treatment for metastatic colorectal cancer (mCRC). Recently, studies have reported the efficacy of TAS-102 combined with bevacizumab in colon cancer patients after standard treatment fails. Here, we evaluated the efficacy and safety of TAS-102 combined with bevacizumab versus TAS-102 as a single agent by a systematic review and a meta-analysis.
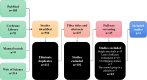
Methods: PubMed, Web of Science and Cochrane libraries were searched. Studies involving bevacizumab combined with TAS-102 in mCRC were included. Study characteristics (author, year of publication, country et al.), efficacy (disease control rate(DCR), progression-free survival(PFS), overall survival(OS)) and adverse effects were extract from studies. Forest plots were created based on Cox model analysis.
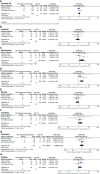
Results: After screening 550 studies, a total of 3 studies were included, which compared the safety and effectiveness of TAS-102 with or without bevacizumab. Analysis based on Cox regression showed that the combined treatment group had advantages in 6-month (OR= 2.93, 95% CI: 1.72 to 5.00, P<0.0001), 12-month(OR= 2.18, 95% CI: 1.24 to 3.81, P=0.006), and 18-month (OR=3.08, 95% CI: 1.34 to 7.12, P=0.008) OS. The combined treatment group demonstrated superiority in 6-month PFS rates (OR= 2.50, 95% CI: 1.18 to 5.31, P=0.02). The incidence of thrombocytopenia in the dual-drug treatment group was higher (OR= 1.96, 95% CI: 1.14 to 3.36 P=0.01). The proportion of serious adverse events were similar in tow groups (OR= 1.01, 95% CI: 0.76 to 1.34 P=0.93).
Conclusion: Bevacizumab combined with TAS-102 could improve the prognosis of patients with mCRC who have failed standard treatment. In terms of side effects, the addition of bevacizumab did not increase serious adverse reactions, but the occurrence of thrombocytopenia was worth noting.
Keywords: TAS-102; bevacizumab; colorectal cancer; meta-analysis 3; survival.
Copyright © 2021 Chen, Qiu, Chen, Wang, Zhu, Pan, Deng, Yang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



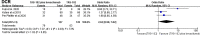
References
-
- Kim ST, Kang JH, Lee J, Lee HW, Oh SY, Jang JS, et al. Capecitabine Plus Oxaliplatin Versus Gemcitabine Plus Oxaliplatin as First-Line Therapy for Advanced Biliary Tract Cancers: A Multicenter, Open-Label, Randomized, Phase III, Noninferiority Trial. Ann Oncol (2019) 30(5):788–95. doi: 10.1093/annonc/mdz058 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

