The circRAB3IP Mediated by eIF4A3 and LEF1 Contributes to Enzalutamide Resistance in Prostate Cancer by Targeting miR-133a-3p/miR-133b/SGK1 Pathway
- PMID: 34868959
- PMCID: PMC8634431
- DOI: 10.3389/fonc.2021.752573
The circRAB3IP Mediated by eIF4A3 and LEF1 Contributes to Enzalutamide Resistance in Prostate Cancer by Targeting miR-133a-3p/miR-133b/SGK1 Pathway
Abstract
An increasing number of studies have shown that circRNAs are closely related to the carcinogenesis and development of prostate cancer (PCa). However, little is known about the effect of the biological functions of circRNAs on the enzalutamide resistance of PCa. Through bioinformatic analysis and experiments, we investigated the expression pattern of circRNAs in enzalutamide-resistant PCa cells. Quantitative real-time PCR was used to detect the expression of circRAB3IP, and plasmids that knock down or overexpress circRAB3IP were used to evaluate its effect on the enzalutamide sensitivity of PCa cells. Mechanistically, we explored the potential regulatory effects of eIF4A3 and LEF1 on the biogenesis of circRAB3IP. Our in vivo and in vitro data indicated that increased expression of circRAB3IP was found in enzalutamide-resistant PCa, and knockdown of circRAB3IP significantly enhanced enzalutamide sensitivity in PCa cells. However, upregulation of circRAB3IP resulted in the opposite effects. Further mechanistic research demonstrated that circRAB3IP could regulate the expression of serum and glucocorticoid-regulated kinase 1 (SGK1) by serving as a sponge that directly targets miR-133a-3p/miR-133b. Then, we showed that circRAB3IP partially exerted its biological functions via SGK1 signaling. Furthermore, we discovered that eIF4A3 and LEF1 might increase circRAB3IP expression in PCa.
Keywords: LEF1; SGK1; circRAB3IP; eIF4A3; enzalutamide resistance.
Copyright © 2021 Chen, Wang, Yang, Keranmu, Zhao, Wu, Han and Xing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
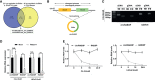

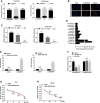


Similar articles
-
Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling.J Exp Clin Cancer Res. 2018 Jul 18;37(1):160. doi: 10.1186/s13046-018-0813-4. J Exp Clin Cancer Res. 2018. PMID: 30021600 Free PMC article.
-
The circRNA circSEPT9 mediated by E2F1 and EIF4A3 facilitates the carcinogenesis and development of triple-negative breast cancer.Mol Cancer. 2020 Apr 7;19(1):73. doi: 10.1186/s12943-020-01183-9. Mol Cancer. 2020. PMID: 32264877 Free PMC article.
-
Enzalutamide-Induced Upregulation of PCAT6 Promotes Prostate Cancer Neuroendocrine Differentiation by Regulating miR-326/HNRNPA2B1 Axis.Front Oncol. 2021 Jun 30;11:650054. doi: 10.3389/fonc.2021.650054. eCollection 2021. Front Oncol. 2021. PMID: 34277403 Free PMC article.
-
LEF1 targeting EMT in prostate cancer invasion is mediated by miR-181a.Am J Cancer Res. 2015 Feb 15;5(3):1124-32. eCollection 2015. Am J Cancer Res. 2015. PMID: 26045991 Free PMC article.
-
Long noncoding RNA SNHG12 indicates the prognosis of prostate cancer and accelerates tumorigenesis via sponging miR-133b.J Cell Physiol. 2020 Feb;235(2):1235-1246. doi: 10.1002/jcp.29039. Epub 2019 Jul 2. J Cell Physiol. 2020. PMID: 31267540
Cited by
-
ARID1A Inactivation Increases Expression of circ0008399 and Promotes Cisplatin Resistance in Bladder Cancer.Curr Med Sci. 2023 Jun;43(3):560-571. doi: 10.1007/s11596-023-2731-8. Epub 2023 May 5. Curr Med Sci. 2023. PMID: 37142816
-
The potential role and mechanism of circRNA/miRNA axis in cholesterol synthesis.Int J Biol Sci. 2023 May 29;19(9):2879-2896. doi: 10.7150/ijbs.84994. eCollection 2023. Int J Biol Sci. 2023. PMID: 37324939 Free PMC article. Review.
-
Role of Serum/Glucocorticoid-Regulated Kinase 1 (SGK1) in Immune and Inflammatory Diseases.Inflammation. 2023 Oct;46(5):1612-1625. doi: 10.1007/s10753-023-01857-8. Epub 2023 Jun 24. Inflammation. 2023. PMID: 37353719 Review.
-
Induro-RT mediated circRNA-sequencing (IMCR-seq) enables comprehensive profiling of full-length and long circular RNAs from low input total RNA.Nucleic Acids Res. 2024 Jul 22;52(13):e55. doi: 10.1093/nar/gkae465. Nucleic Acids Res. 2024. PMID: 38850158 Free PMC article.
-
The crosstalk between alternative splicing and circular RNA in cancer: pathogenic insights and therapeutic implications.Cell Mol Biol Lett. 2024 Nov 16;29(1):142. doi: 10.1186/s11658-024-00662-x. Cell Mol Biol Lett. 2024. PMID: 39550559 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

