Early Death and Survival of Patients With Acute Promyelocytic Leukemia in ATRA Plus Arsenic Era: A Population-Based Study
- PMID: 34868978
- PMCID: PMC8637823
- DOI: 10.3389/fonc.2021.762653
Early Death and Survival of Patients With Acute Promyelocytic Leukemia in ATRA Plus Arsenic Era: A Population-Based Study
Abstract
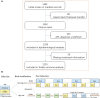
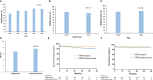
Most randomized trials for acute promyelocytic leukemia (APL) have investigated highly selected patients under idealized conditions, and the findings need to be validated in the real world. We conducted a population-based study of all APL patients in Zhejiang Province, China, with a total population of 82 million people, to assess the generalization of all-trans retinoic acid (ATRA) and arsenic as front-line treatment. The outcomes of APL patients were also analyzed. Between January 2015 and December 2019, 1,233 eligible patients were included in the final analysis. The rate of ATRA and arsenic as front-line treatment increased steadily from 66.2% in 2015 to 83.3% in 2019, with no difference among the size of the center (≥5 or <5 patients per year, p = 0.12) or age (≥60 or <60 years, p = 0.35). The early death (ED) rate, defined as death within 30 days after diagnosis, was 8.2%, and the 3-year overall survival (OS) was 87.9% in the whole patient population. Age (≥60 years) and white blood cell count (>10 × 109/L) were independent risk factors for ED and OS in the multivariate analysis. This population-based study showed that ATRA and arsenic as front-line treatment are widely used under real-world conditions and yield a low ED rate and a high survival rate, which mimic the results from clinical trials, thereby supporting the wider application of APL guidelines in the future.
Keywords: ATRA; acute promyelocytic leukemia; arsenic; early death; survival.
Copyright © 2021 Zhu, Ma, Yu, Ouyang, Luo, Pei, Xu, Hu, Mo, Xu, Lan, Shen, Shou, Qian, Feng, Zhao, Jiang, Hu, Zhang, Qian, Wu, Wu, Qiu, Li, Lang, Chen, Chen, Guo, Cao, Jiang, Xia, Le, Zhao, Huang, Zhang, Lv, Hua, Hong, Zheng, Wang, Hu, Chen, Zhang, Tao, Xie, Kuang, Luo, Su, Guo, Wu, Jiang, Zhang, Zhang, Chen, Xu, Guo, Tu, Hu, Yan, Yao, Lou, Jin and the APL Cooperative Group of Zhejiang Province.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

