Prognostic Potential of Liver Enzymes in Patients With COVID-19 at the Leishenshan Hospital in Wuhan
- PMID: 34869048
- PMCID: PMC8635154
- DOI: 10.3389/fcimb.2021.636999
Prognostic Potential of Liver Enzymes in Patients With COVID-19 at the Leishenshan Hospital in Wuhan
Abstract
Background: Coronavirus disease 2019 (COVID-19) has evolved into a pandemic. We hypothesized that biochemical indicators of liver function may help determine the prognosis of COVID-19 patients.
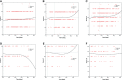
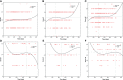
Methods: Patient information was collected from the Wuhan-Leishenshan hospital. Logistic and Cox regression analyses, Kaplan-Meier curves, and Curve fitting were used to determine the correlation between elevated levels of aspartate transaminase (AST), alanine transaminase (ALT), and AST/ALT and severity of disease/mortality.
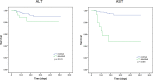
Results: Logistic and Cox regression analyses and Kaplan-Meier survival curves showed that COVID-19 progression correlated with elevated levels of AST and AST/ALT. The odds ratios for elevated levels of AST and AST/ALT in patients were 0.818 (95% confidence interval [CI]: 0.274-2.441, P = 0.035) and 2.055 (95% CI: 1.269-3.327, P = 0.003), respectively; the hazard ratios were 4.195 (95% CI: 1.219-14.422, P = 0.023) and 3.348 (95% CI: 1.57-7.139, P = 0.002), respectively. The Kaplan-Meier survival curves demonstrated that patients with elevated AST and AST/ALT levels had a higher risk of developing severe COVID-19.
Conclusion: Elevated AST and AST/ALT levels correlated with severity of COVID-19 and mortality. Liver function tests may help clinicians in determining the prognosis of patients undergoing treatment for COVID-19.
Keywords: ALT - alanine transaminase; AST - aspartate transaminase; AST/ALT; COVID-19; aspartate aminotransferase/alanine aminotransferase; pneumonia.
Copyright © 2021 Liu, Hu, Li, Xia, Gong, Li, Wu, Yi, Huang, Wu, Guo and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Impact of Liver Chemistries on Respiratory Failure among Hemodialysis Patients with COVID-19 during the Omicron Wave.Intern Med. 2023 Sep 15;62(18):2617-2625. doi: 10.2169/internalmedicine.2115-23. Epub 2023 Jul 5. Intern Med. 2023. PMID: 37407459 Free PMC article.
-
Association of procalcitonin levels with the progression and prognosis of hospitalized patients with COVID-19.Int J Med Sci. 2020 Sep 9;17(16):2468-2476. doi: 10.7150/ijms.48396. eCollection 2020. Int J Med Sci. 2020. PMID: 33029089 Free PMC article.
-
Preoperative aspartate transaminase/alanine transaminase ratio as a prognostic biomarker in primary non-muscle-invasive bladder cancer: a propensity score-matched study.BMC Urol. 2021 Sep 27;21(1):136. doi: 10.1186/s12894-021-00901-9. BMC Urol. 2021. PMID: 34579695 Free PMC article.
-
Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: systematic review with meta-analysis.Eur Rev Med Pharmacol Sci. 2020 Dec;24(24):13072-13088. doi: 10.26355/eurrev_202012_24215. Eur Rev Med Pharmacol Sci. 2020. PMID: 33378061
-
Liver injury in patients with severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis.Eur J Gastroenterol Hepatol. 2021 Sep 1;33(9):1194-1200. doi: 10.1097/MEG.0000000000001827. Eur J Gastroenterol Hepatol. 2021. PMID: 32796355
Cited by
-
COVID-19 Vaccination in Patients with Chronic Liver Disease.Viruses. 2022 Dec 13;14(12):2778. doi: 10.3390/v14122778. Viruses. 2022. PMID: 36560782 Free PMC article. Review.
-
Hemostatic and liver function parameters as COVID-19 severity markers.Narra J. 2024 Apr;4(1):e178. doi: 10.52225/narra.v4i1.178. Epub 2024 Mar 2. Narra J. 2024. PMID: 38798852 Free PMC article.
-
Performance of Derived Laboratory Biomarkers with Regard to 30-Day Mortality in Kidney Transplant Recipients with COVID-19.Life (Basel). 2022 Dec 9;12(12):2068. doi: 10.3390/life12122068. Life (Basel). 2022. PMID: 36556433 Free PMC article.
-
Prognostic Value of Transaminases and Bilirubin Levels at Admission to Hospital on Disease Progression and Mortality in Patients with COVID-19-An Observational Retrospective Study.Pathogens. 2022 Jun 6;11(6):652. doi: 10.3390/pathogens11060652. Pathogens. 2022. PMID: 35745506 Free PMC article.
-
Safety and efficacy of tixagevimab/cilgavimab for pre-exposure prophylaxis in kidney transplant recipients: a multicenter retrospective cohort study.J Nephrol. 2024 Jul;37(6):1539-1550. doi: 10.1007/s40620-024-01889-9. Epub 2024 May 23. J Nephrol. 2024. PMID: 38780697 Free PMC article.
References
-
- Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A., et al. . (2020). Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv 2020.02.03.931766. doi: 10.1101/2020.02.03.931766 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

