A Novel Calix[4]Crown-Based 1,3,4-Oxadiazole as a Fluorescent Chemosensor for Copper(II) Ion Detection
- PMID: 34869207
- PMCID: PMC8632693
- DOI: 10.3389/fchem.2021.766442
A Novel Calix[4]Crown-Based 1,3,4-Oxadiazole as a Fluorescent Chemosensor for Copper(II) Ion Detection
Abstract
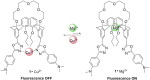
The synthesis and characterization of a novel florescent chemosensor 1 with two different types of cationic binding sites have been reported in this work, which is a calix[4]crown derivative in 1,3-alternate conformation bearing two 2-phenyl-5-(4-dimethylaminopyenyl)-1,3,4-oxadiazole units. The recognition behaviors of 1 in dichloromethane/acetonitrile solution to alkali metal ions (Na+ and K+), alkaline earth metal ions (Mg2+ and Ca2+), and transition metal ions (Co2+, Ni2+, Zn2+, Cd2+, Cu2+, Mn2+, and Ag+) have been investigated by UV-Vis and fluorescence spectra. The fluorescence of 1 might be quenched selectively by Cu2+ due to the photo-induced electron transfer mechanism, and the quenched emission from 1 could be partly revived by the addition of Ca2+ or Mg2+; thus, the receptor 1 might be worked as an on-off switchable fluorescent chemosensor triggered by metal ion exchange.
Keywords: 1,3,4-oxadiazole; 1,3-alternate conformation; calix[4]crown; copper (II) detection; fluorescent chemosensor.
Copyright © 2021 Sun, Du, Zhang and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Becke A. D. (1993). Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 98, 5648. 10.1063/1.464913 - DOI
-
- Cao X., Li Y., Gao A., Yu Y., Zhou Q., Chang X. (2019). Multifunctional Fluorescent Naphthalimide Self-Assembly System for the Detection of Cu2+ and K+ and Continuous Sensing of Organic Amines and Gaseous Acids. J. Mater. Chem. C 7, 10589–10597. 10.1039/c9tc03243f - DOI
-
- Chen Y.-J., Chen M.-Y., Lee K.-T., Shen L.-C., Hung H.-C., Niu H.-C., et al. (2020). 1,3-Alternate Calix[4]arene Functionalized with Pyrazole and Triazole Ligands as a Highly Selective Fluorescent Sensor for Hg2+ and Ag+ Ions. Front. Chem. 6, 593261. 10.3389/fchem.2019.59326110.3389/fchem.2020.593261 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

