Human Sperm Remain Motile After a Temporary Energy Restriction but do Not Undergo Capacitation-Related Events
- PMID: 34869380
- PMCID: PMC8633110
- DOI: 10.3389/fcell.2021.777086
Human Sperm Remain Motile After a Temporary Energy Restriction but do Not Undergo Capacitation-Related Events
Abstract
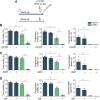
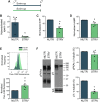
To acquire fertilization competence, mammalian sperm must undergo several biochemical and physiological modifications known as capacitation. Despite its relevance, the metabolic pathways that regulate the capacitation-related events, including the development of hyperactivated motility, are still poorly described. Previous studies from our group have shown that temporary energy restriction in mouse sperm enhanced hyperactivation, in vitro fertilization, early embryo development and pregnancy rates after embryo transfer, and it improved intracytoplasmic sperm injection results in the bovine model. However, the effects of starvation and energy recovery protocols on human sperm function have not yet been established. In the present work, human sperm were incubated for different periods of time in medium containing glucose, pyruvate and lactate (NUTR) or devoid of nutrients for the starving condition (STRV). Sperm maintained in STRV displayed reduced percentages of motility and kinematic parameters compared to cells incubated in NUTR medium. Moreover, they did not undergo hyperactivation and showed reduced levels of ATP, cAMP and protein tyrosine phosphorylation. Similar to our results with mouse sperm, starvation induced increased intracellular Ca2+ concentrations. Starved human sperm were capable to continue moving for more than 27 h, but the incubation with a mitochondrial uncoupler or inhibitors of oxidative phosphorylation led to a complete motility loss. When exogenous nutrients were added back (sperm energy recovery (SER) treatment), hyperactivated motility was rescued and there was a rise in sperm ATP and cAMP levels in 1 min, with a decrease in intracellular Ca2+ concentration and no changes in sperm protein tyrosine phosphorylation. The finding that human sperm can remain motile for several hours under starvation due to mitochondrial use of endogenous metabolites implies that other metabolic pathways may play a role in sperm energy production. In addition, full recovery of motility and other capacitation parameters of human sperm after SER suggests that this treatment might be used to modulate human sperm fertilizing ability in vitro.
Keywords: ATP; capacitation; glucose; glycolysis; metabolism; oxidative phosphorylation; pyruvate/lactate; sperm motility.
Copyright © 2021 Marín-Briggiler, Luque, Gervasi, Oscoz-Susino, Sierra, Mondillo, Salicioni, Krapf, Visconti and Buffone.
Conflict of interest statement
PEV and AMS own equity interest in Sperm Capacitation Technologies Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Albarracín J. L., Fernández J. M., Ballester J., Rauch M. C., Quintero-Moreno A., Peña A., et al. (2004). Gluconeogenesis-Linked Glycogen Metabolism Is Important in the Achievement of In Vitro Capacitation of Dog Spermatozoa in a Medium without Glucose1. Biol. Reprod. 71, 1437–1445. 10.1095/biolreprod.104.029041 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

