Biochemical evaluation and molecular docking assessment of Cymbopogon citratus as a natural source of acetylcholine esterase (AChE)- targeting insecticides
- PMID: 34869921
- PMCID: PMC8626657
- DOI: 10.1016/j.bbrep.2021.101175
Biochemical evaluation and molecular docking assessment of Cymbopogon citratus as a natural source of acetylcholine esterase (AChE)- targeting insecticides
Abstract
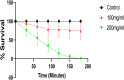
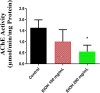
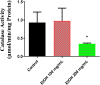
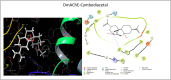
Acetylcholinesterase (AChE) has been an effective target for insecticide development which is a very important aspect of the global fight against insect-borne diseases. The drastic reduction in the sensitivity of insects to AChE-targeting insecticides like organophosphates and carbamates have increased the need for insecticides of natural origin. In this study, we used Drosophila melanogaster as a model to investigate the insecticidal and AChE inhibitory potentials of Cymbopogon citratus and its bioactive compounds. Flies were exposed to 100 and 200 mg/mL C. citratus leaf extract for a 3-h survival assay followed by 45 min exposure for negative geotaxis and biochemical assays. Molecular docking analysis of 45 bioactive compounds of the plant was conducted against Drosophila melanogaster AChE (DmAChE). Exposure to C. citratus significantly reduced the survival rate of flies throughout the exposure period and this was accompanied by a significant decrease in percentage negative geotaxis, AChE activity, catalase activity, total thiol level and a significant increase in glutathione-S-transferase (GST) activity. The bioactive compounds of C. citratus showed varying levels of binding affinities for the enzyme. (+)-Cymbodiacetal scored highest (-9.407 kcal/mol) followed by proximadiol (-8.253 kcal/mol), geranylacetone (-8.177 kcal/mol), and rutin (-8.148 kcal/mol). The four compounds occupied the same binding pocket and interacted with important active site amino acid residues as the co-crystallized ligand (1qon). These compounds could be responsible for the insecticidal and AChE inhibitory potentials of C. citratus and they could be further explored in the development of AChE-targeting insecticides.
Keywords: Acetylcholinesterase; Binding affinity; Cymbopogon citratus; Drosophila melanogaster.
© 2021 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Johnson T.O., Abolaji A.O., Omale S., Longdet Y.I., Kutshik J.R., Oyetayo O.B., et al. Benzo[a]pyrene and Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide induced locomotor and reproductive senescence and altered biochemical parameters of oxidative damage in Canton-S Drosophila melanogaster. Toxicol. Rep. 2021;8:571–580. doi: 10.1016/j.toxrep.2021.03.001. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

