Construction of Spirooxindole Analogues Engrafted with Indole and Pyrazole Scaffolds as Acetylcholinesterase Inhibitors
- PMID: 34869980
- PMCID: PMC8637602
- DOI: 10.1021/acsomega.1c03978
Construction of Spirooxindole Analogues Engrafted with Indole and Pyrazole Scaffolds as Acetylcholinesterase Inhibitors
Abstract
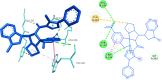
Twenty-five new hits of spirooxindole analogs 8a-y engrafted with indole and pyrazole scaffolds were designed and constructed via a [3+2]cycloaddition (32CA) reaction starting from three components: new chalcone-based indole and pyrazole scaffolds 5a-d, substituted isatins 6a-c, and secondary amines 7a-d. The potency of the compounds were assessed in modulating cholinesterase (AChE) activity using Ellman's method. Compounds 8i and 8y showed the strongest acetylcholine esterase inhibition (AChEI) with IC50 values of 24.1 and 27.8 μM, respectively. Molecular docking was used to study their interaction with the active site of hAChE.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
LinkOut - more resources
Full Text Sources

