A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India
- PMID: 34870133
- PMCID: PMC8629682
- DOI: 10.1016/j.eclinm.2021.101218
A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India
Abstract
Background: This phase 2/3 immunobridging study evaluated the safety and immunogenicity of the ChAdOx1 nCoV-19 Coronavirus Vaccine (Recombinant) (SII-ChAdOx1 nCoV-19), manufactured in India at the Serum Institute of India Pvt Ltd (SIIPL), following technology transfer from the AstraZeneca.
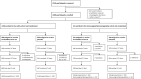
Methods: This participant-blind, observer-blind study randomised participants 3:1 to SII-ChAdOx1 nCoV-19 or AZD1222 (ChAdOx1 nCoV-19) (immunogenicity/reactogenicity cohort) and 3:1 to SII-ChAdOx1 nCoV-19 or placebo (safety cohort). The study participants were enrolled from 14 hospitals across India between August 25 and October 31, 2020. Two doses of study products were given 4 weeks apart. The primary objectives were to demonstrate non-inferiority of SII-ChAdOx1 nCoV-19 to AZD1222 in terms of geometric mean titre (GMT) ratio of anti-SARS-CoV-2 spike IgG antibodies 28 days after the second dose (defined as lower limit of 95% CI >0·67) and to determine the incidence of serious adverse events (SAEs) causally related to SII-ChAdOx1 nCoV-19. The anti-spike IgG response was assessed using a multiplexed electrochemiluminescence-based immunoassay. Safety follow-up continued until 6 months after first dose. Trial registration: CTRI/2020/08/027170.
Findings: 1601 participants were enrolled: 401 to the immunogenicity/reactogenicity cohort and 1200 to the safety cohort. After two doses, seroconversion rates for anti-spike IgG antibodies were more than 98·0% in both the groups. SII-ChAdOx1 nCoV-19 was non-inferior to AZD1222 (GMT ratio 0·98; 95% CI 0·78-1·23). SAEs were reported in ≤ 2·0% participants across the three groups; none were causally related. A total of 34 SARS-CoV-2 infections were reported; of which 6 occurred more than 2 weeks after the second dose; none were severe.
Interpretation: SII-ChAdOx1 nCoV-19 has a non-inferior immune response compared to AZD1222 and an acceptable safety/reactogenicity profile. Pharmacovigilance should be maintained to detect any safety signals.
Funding: SIIPL funded the contract research organisation and laboratory costs, while the site costs were funded by the Indian Council of Medical Research. The study vaccines were supplied by SIIPL and AstraZeneca.
Keywords: AZD1222; COVID-19; Immunogenicity; SII-ChAdOx1 nCoV-19; Safety.
© 2021 The Authors.
Conflict of interest statement
PSK, CB, AD, MG, US, DK, and BG are employees of SIIPL. JV and EJK are employees of AstraZeneca. All other authors declare no competing interests.
Figures

References
-
- World Health Organization (WHO). WHO Director-General's opening remarks at the media briefing on COVID-19. March 11, 2020. https://www.who.int/director-general/speeches/detail/who-director-genera... (accessed Jul 5, 2021).
LinkOut - more resources
Full Text Sources
Miscellaneous

