Protective effects of cerium oxide nanoparticles in non-alcoholic fatty liver disease (NAFLD) and carbon tetrachloride-induced liver damage in rats: Study on intestine and liver
- PMID: 34870139
- PMCID: PMC8626579
- DOI: 10.1016/j.metop.2021.100151
Protective effects of cerium oxide nanoparticles in non-alcoholic fatty liver disease (NAFLD) and carbon tetrachloride-induced liver damage in rats: Study on intestine and liver
Abstract
Background and aims: Nanoparticles could represent a therapeutic approach for the treatment of various diseases. It has been reported that cerium oxide nanoparticles (CeO2 NPs) have potential useful effects. Therefore, we aimed to examine the protective effects of the CeO2 NPs in two models of liver injury, non-alcoholic fatty liver disease (NAFLD) and carbon tetrachloride (CCl4)-induced liver fibrosis, in rats.
Methods: In this experimental study, male rats were randomly divided into different experimental groups including: Experiment 1; group1: healthy rats received normal saline, 2: CCl4 group, 3: CCl4 + nanoparticle. Experiment 2; group1: healthy rats received chow diet, 2: NAFLD group, 3: NAFLD + nanoparticle. The oxidative stress markers were determined in the liver and intestine. Tumor necrosis factor-α (TNF-α) levels were measured by ELISA. Histopathological changes of liver and intestine were evaluated by light microspore.
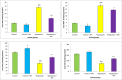
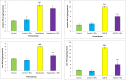
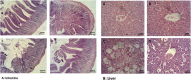
Results: Total antioxidant capacity (TAC) and glutathione (GSH) levels significantly decreased, while malondialdehyde (MDA) and total oxidant status (TOS) were significantly increased in the liver, and intestine of the NAFLD and CCl4 group compared with control rats. However, the use of nanoparticles significantly normalized these markers. The levels of the TNF-α were significantly reduced in the nanoparticle group as compared with NAFLD model and CCl4-treated rats. CeO2 NPs also normalized the liver and intestinal histological changes.
Conclusions: Our finding revealed that CeO2 NPs has potential protective effects by increasing antioxidant activity, and reducing inflammation.
Keywords: Carbon tetrachloride; Cerium oxide nanoparticles; Inflammation; Liver injury; Rats.
© 2021 The Authors. Published by Elsevier Inc.
Figures





Similar articles
-
Multi-organ Toxicity Attenuation by Cerium Oxide and Yttrium Oxide Nanoparticles: Comparing the Beneficial Effects on Tissues Oxidative Damage Induced by Sub-acute Exposure to Diazinon.Pharm Nanotechnol. 2020;8(3):225-238. doi: 10.2174/2211738508666200808135226. Pharm Nanotechnol. 2020. PMID: 32767961
-
Aortic Oxidative Stress, Inflammation and DNA Damage Following Pulmonary Exposure to Cerium Oxide Nanoparticles in a Rat Model of Vascular Injury.Biomolecules. 2019 Aug 17;9(8):376. doi: 10.3390/biom9080376. Biomolecules. 2019. PMID: 31426470 Free PMC article.
-
Is human umbilical cord mesenchymal stem cell-derived conditioned medium effective against oxidative and inflammatory status in CCl4-induced acute liver injury?Life Sci. 2022 Sep 15;305:120730. doi: 10.1016/j.lfs.2022.120730. Epub 2022 Jun 23. Life Sci. 2022. PMID: 35753436
-
The protective effect of chrysin against carbon tetrachloride-induced kidney and liver tissue damage in rats.Int J Vitam Nutr Res. 2021 Sep;91(5-6):427-438. doi: 10.1024/0300-9831/a000653. Epub 2020 Apr 30. Int J Vitam Nutr Res. 2021. PMID: 32349632
-
Cerium oxide nanoparticles modulate liver X receptor and short heterodimer partner, and attenuate liver steatosis and steatohepatitis in a rat model of postmenopausal obesity.Gen Physiol Biophys. 2022 Sep;41(5):431-446. doi: 10.4149/gpb_202235. Gen Physiol Biophys. 2022. PMID: 36222341
Cited by
-
Effects of cerium oxide nanoparticle on liver oxidative stress and morphological changes in opium withdrawal rats.Toxicol Rep. 2025 Apr 9;14:102025. doi: 10.1016/j.toxrep.2025.102025. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 40290115 Free PMC article.
-
Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine.Pharmaceuticals (Basel). 2025 Jan 24;18(2):154. doi: 10.3390/ph18020154. Pharmaceuticals (Basel). 2025. PMID: 40005968 Free PMC article. Review.
-
Effectiveness of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease Evolution Using In Vivo and In Vitro Studies: A Systematic Review.Int J Mol Sci. 2023 Oct 29;24(21):15728. doi: 10.3390/ijms242115728. Int J Mol Sci. 2023. PMID: 37958712 Free PMC article.
-
Editorial: Special issue: Non-alcoholic fatty liver disease: From molecular basis to therapeutic advances.Metabol Open. 2023 Jan 13;17:100229. doi: 10.1016/j.metop.2023.100229. eCollection 2023 Mar. Metabol Open. 2023. PMID: 36686606 Free PMC article. No abstract available.
-
Silver Nanoparticles Synthesized from Enicostemma littorale Exhibit Gut Tight Junction Restoration and Hepatoprotective Activity via Regulation of the Inflammatory Pathway.Pharmaceutics. 2025 Jul 9;17(7):895. doi: 10.3390/pharmaceutics17070895. Pharmaceutics. 2025. PMID: 40733103 Free PMC article.
References
-
- Ong J.P., Younossi Z.M. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16. [vii] - PubMed
-
- Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. - PubMed
-
- Wiwanitkit V. Alcoholic consumption behavior and death due to swine flu. Egypt J Chest Dis Tuberc. 2016;65:169–171.
-
- Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. - PubMed
LinkOut - more resources
Full Text Sources

