Broad-spectrum therapeutics: A new antimicrobial class
- PMID: 34870144
- PMCID: PMC8035643
- DOI: 10.1016/j.crphar.2020.100011
Broad-spectrum therapeutics: A new antimicrobial class
Abstract
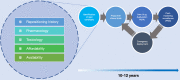
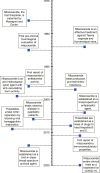
There are currently no emergency treatments for pandemics, yet drug repositioning has emerged as the foremost treatment development strategy for COVID-19, with an aim to identify successful antiviral therapeutics from safe, non-antiviral candidates. These therapeutics include antibiotics such as azithromycin and the antiparasitic nitazoxanide, both of which exhibit antiviral activity. Broad-spectrum therapeutics (BSTs) are a class of antimicrobials active against multiple pathogen types. Establishment of a developmental framework for BSTs will markedly improve global preparedness for future health emergencies.
Keywords: Antimicrobial; Broad-spectrum therapeutic; COVID-19; Drug repositioning.
Crown Copyright © 2020 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Allison M. NCATS launches drug repurposing program. Nat. Biotechnol. 2012;30(7):571–572. - PubMed
-
- Avery A., Anderson C., Bond C., et al. Evaluation of patient reporting of adverse drug reactions to the UK's ‘Yellow Card scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol. Assess. 2011;15:1–234. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

