Recent progress in the development of potential drugs against SARS-CoV-2
- PMID: 34870155
- PMCID: PMC8437701
- DOI: 10.1016/j.crphar.2021.100057
Recent progress in the development of potential drugs against SARS-CoV-2
Abstract
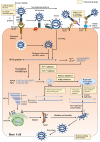
SARS-CoV-2, a newly emerged and highly pathogenic coronavirus, is identified as the causal agent of Coronavirus Disease (2019) (COVID-19) in the late December 2019, in China. The virus has rapidly spread nationwide and spilled over to the other countries around the globe, resulting in more than 120 million infections and 2.6 million deaths until the time of this review. Unfortunately, there are still no specific drugs available against this disease, and it is very necessary to call upon more scientists to work together to stop a further spread. Hence, the recent progress in the development of drugs may help scientific community quickly understand current research status and further develop new effective drugs. Herein, we summarize the cellular entry and replication process of this virus and discuss the recent development of potential viral based drugs that target bio-macromolecules in different stages of the viral life cycle, especially S protein, 3CLPro, PLPro, RdRp and helicase.
Keywords: Coronavirus; Entry inhibitors; Protease inhibitors; SARS-CoV-2.
© 2021 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

