An updated review on potential therapeutic drug candidates, vaccines and an insight on patents filed for COVID-19
- PMID: 34870158
- PMCID: PMC8498785
- DOI: 10.1016/j.crphar.2021.100063
An updated review on potential therapeutic drug candidates, vaccines and an insight on patents filed for COVID-19
Abstract
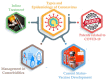
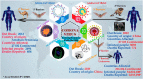
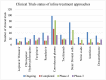
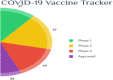
The outbreak of COVID-19 was recognized in December 2019 in China and as of October5th, the pandemic was swept through 216 countries and infected around 34,824,108 individuals, thus posing an unprecedented threat to world's health and economy. Several researchers reported that, a significant mutation in membrane proteins and receptor binding sites of preceding severe acute respiratory syndrome coronavirus (SARS-CoV) to turned as novel SARS-CoV-2 virus and disease was named as COVID-19 (Coronavirus disease 2019). Unfortunately, there is no specific treatment available for COVID-19 patients. The lessons learned from the past management of SARS-CoV and other pandemics, have provided some insights to treat COVID-19. Currently, therapies like anti-viral treatment, immunomodulatory agents, plasma transfusion and supportive intervention etc., are using to treat the COVID-19. Few of these were proven to provide significant therapeutic benefits in treating the COVID-19, however no drug is approved by the regulatory agencies. As the fatality rate is high in patients with comorbid conditions, we have also enlightened the current in-line treatment therapies and specific treatment strategies in comorbid conditions to combat the emergence of COVID-19. In addition, pharmaceutical, biological companies and research institutions across the globe have begun to develop thesafe and effective vaccine for COVID-19. Globally around 170 teams of researchers are racing to develop the COVID-19 vaccine and here we have discussed about their current status of development. Furthermore, recent patents filed in association with COVID-19 was elaborated. This can help many individuals, researchers or health workers, in applying these principles for diagnosis/prevention/management/treatment of the current pandemic.
Keywords: Clinical trials; Covid-19; Inline treatment; Patents; Vaccines.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interestsor personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Current status of potential therapeutic candidates for the COVID-19 crisis.Brain Behav Immun. 2020 Jul;87:59-73. doi: 10.1016/j.bbi.2020.04.046. Epub 2020 Apr 22. Brain Behav Immun. 2020. PMID: 32334062 Free PMC article. Review.
-
Insights into Novel Coronavirus Disease 2019 (COVID-19): Current Understanding, Research, and Therapeutic Updates.Recent Pat Biotechnol. 2022;16(1):35-63. doi: 10.2174/1872208315666210805152122. Recent Pat Biotechnol. 2022. PMID: 34353275 Review.
-
A Scoping Insight on Potential Prophylactics, Vaccines and Therapeutic Weaponry for the Ongoing Novel Coronavirus (COVID-19) Pandemic- A Comprehensive Review.Front Pharmacol. 2021 Feb 26;11:590154. doi: 10.3389/fphar.2020.590154. eCollection 2020. Front Pharmacol. 2021. PMID: 33815095 Free PMC article. Review.
-
Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19).J Microbiol Biotechnol. 2020 Mar 28;30(3):313-324. doi: 10.4014/jmb.2003.03011. J Microbiol Biotechnol. 2020. PMID: 32238757 Free PMC article. Review.
-
The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem.J Biol Regul Homeost Agents. 2021 Jan-Feb;35(1):1-4. doi: 10.23812/21-3-E. J Biol Regul Homeost Agents. 2021. PMID: 33377359
Cited by
-
Portal Vein and Mesenteric Artery Thrombosis Following the Administration of an Ad26.COV2-S Vaccine-First Case from Romania: A Case Report.Vaccines (Basel). 2022 Nov 18;10(11):1950. doi: 10.3390/vaccines10111950. Vaccines (Basel). 2022. PMID: 36423045 Free PMC article.
-
Dynamics of specific antibodies in COVID-19 patients after recovery.Epidemiol Infect. 2022 Mar 22;150:1-27. doi: 10.1017/S0950268822000528. Online ahead of print. Epidemiol Infect. 2022. PMID: 35314019 Free PMC article.
-
SARS-CoV-2 variants and vulnerability at the global level.J Med Virol. 2022 Jul;94(7):2986-3005. doi: 10.1002/jmv.27717. Epub 2022 Apr 5. J Med Virol. 2022. PMID: 35277864 Free PMC article. Review.
-
COVID-19 therapy, from lung disease to systemic disorder.Curr Res Pharmacol Drug Discov. 2022;3:100099. doi: 10.1016/j.crphar.2022.100099. Epub 2022 Apr 1. Curr Res Pharmacol Drug Discov. 2022. PMID: 35382154 Free PMC article. No abstract available.
-
Pharmaceutical/Biomedical Applications of Electrospun Nanofibers - Comprehensive Review, Attentive to Process Parameters and Patent Landscape.Pharm Nanotechnol. 2024;12(5):412-427. doi: 10.2174/2211738511666230911163249. Pharm Nanotechnol. 2024. PMID: 37702161 Review.
References
-
- Ahn D.G., Shin H.J., Kim M.H., Lee S., Kim H.S., Myoung J., Kim B.T., Kim S.J. vol. 30. Korean Society for Microbiology and Biotechnology; 2020. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) pp. 313–324. (Journal of Microbiology and Biotechnology). 3. - DOI - PMC - PubMed
-
- Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Prato S. Del. Review COVID-19 in people with diabetes: understanding the reasons for worse outcomes. 2017. Www.Thelancet.Com/Diabetes-Endocrinology. 8. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

