Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent
- PMID: 34870327
- PMCID: PMC8647093
- DOI: 10.1002/14651858.CD011300.pub3
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent
Abstract
Background: Non-small cell lung cancer (NSCLC) is the most common lung cancer, accounting for approximately 80% to 85% of all cases. For people with localised NSCLC (stages I to III), it has been speculated that immunotherapy may be helpful for reducing postoperative recurrence rates, or improving the clinical outcomes of current treatment for unresectable tumours. This is an update of a Cochrane Review first published in 2017 and it includes two new randomised controlled trials (RCTs).
Objectives: To assess the effectiveness and safety of immunotherapy (excluding checkpoint inhibitors) among people with localised NSCLC of stages I to III who received curative intent of radiotherapy or surgery.
Search methods: We searched the following databases (from inception to 19 May 2021): CENTRAL, MEDLINE, Embase, CINAHL, and five trial registers. We also searched conference proceedings and reference lists of included trials.
Selection criteria: We included RCTs conducted in adults (≥ 18 years) diagnosed with NSCLC stage I to III after surgical resection, and those with unresectable locally advanced stage III NSCLC receiving radiotherapy with curative intent. We included participants who underwent primary surgical treatment, postoperative radiotherapy or chemoradiotherapy if the same strategy was provided for both intervention and control groups.
Data collection and analysis: Two review authors independently selected eligible trials, assessed risk of bias, and extracted data. We used survival analysis to pool time-to-event data, using hazard ratios (HRs). We used risk ratios (RRs) for dichotomous data, and mean differences (MDs) for continuous data, with 95% confidence intervals (CIs). Due to clinical heterogeneity (immunotherapeutic agents with different underlying mechanisms), we combined data by applying random-effects models.
Main results: We included 11 RCTs involving 5128 participants (this included 2 new trials with 188 participants since the last search dated 20 January 2017). Participants who underwent surgical resection or received curative radiotherapy were randomised to either an immunotherapy group or a control group. The immunological interventions were active immunotherapy Bacillus Calmette-Guérin (BCG) adoptive cell transfer (i.e. transfer factor (TF), tumour-infiltrating lymphocytes (TIL), dendritic cell/cytokine-induced killer (DC/CIK), antigen-specific cancer vaccines (melanoma-associated antigen 3 (MAGE-A3) and L-BLP25), and targeted natural killer (NK) cells. Seven trials were at high risk of bias for at least one of the risk of bias domains. Three trials were at low risk of bias across all domains and one small trial was at unclear risk of bias as it provided insufficient information. We included data from nine of the 11 trials in the meta-analyses involving 4863 participants. There was no evidence of a difference between the immunotherapy agents and the controls on any of the following outcomes: overall survival (HR 0.94, 95% CI 0.84 to 1.05; P = 0.27; 4 trials, 3848 participants; high-quality evidence), progression-free survival (HR 0.94, 95% CI 0.86 to 1.03; P = 0.19; moderate-quality evidence), adverse events (RR 1.12, 95% CI 0.97 to 1.28; P = 0.11; 4 trials, 4126 evaluated participants; low-quality evidence), and severe adverse events (RR 1.14, 95% CI 0.92 to 1.40; 6 trials, 4546 evaluated participants; low-quality evidence). Survival rates at different time points showed no evidence of a difference between immunotherapy agents and the controls. Survival rate at 1-year follow-up (RR 1.02, 95% CI 0.96 to 1.08; I2 = 57%; 7 trials, 4420 participants; low-quality evidence), 2-year follow-up (RR 1.02, 95% CI 0.93 to 1.12; 7 trials, 4420 participants; moderate-quality evidence), 3-year follow-up (RR 0.99, 95% CI 0.90 to 1.09; 7 trials, 4420 participants; I2 = 22%; moderate-quality evidence) and at 5-year follow-up (RR 0.98, 95% CI 0.86 to 1.12; I2 = 0%; 7 trials, 4389 participants; moderate-quality evidence). Only one trial reported overall response rates. Two trials provided health-related quality of life results with contradicting results. AUTHORS' CONCLUSIONS: Based on this updated review, the current literature does not provide evidence that suggests a survival benefit from adding immunotherapy (excluding checkpoint inhibitors) to conventional curative surgery or radiotherapy, for people with localised NSCLC (stages I to III). Several ongoing trials with immune checkpoints inhibitors (PD-1/PD-L1) might bring new insights into the role of immunotherapy for people with stages I to III NSCLC.
Trial registration: ClinicalTrials.gov NCT00409188 NCT00960115 NCT00290355 NCT00480025 NCT01015443.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
JZ: declares no conflict of interest
YY: declares no conflict of interest
XW: declares no conflict of interest
DY: declares no conflict of interest
RL: declares no conflict of interest
WC: declares no conflict of interest
CS: declares no conflict of interest
HS: declares no conflict of interest
Figures

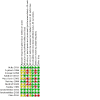










Update of
-
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent.Cochrane Database Syst Rev. 2017 Dec 16;12(12):CD011300. doi: 10.1002/14651858.CD011300.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Dec 6;12:CD011300. doi: 10.1002/14651858.CD011300.pub3. PMID: 29247502 Free PMC article. Updated.
References
References to studies included in this review
Butts 2014 {published data only}
-
- Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer(START): a randomised, double-blind, phase 3 trial. Lancet Oncolology 2014;15(1):59-68. - PubMed
Fujisawa 1996 {published data only}
-
- Fujisawa T, Yamaguchi Y. Postoperative immunostimulation after complete resection improves survival of patients with stage I non-small cell lung carcinoma. Cancer 1996;78(9):1892-8. - PubMed
Giovanni 1996 {published data only}
-
- Giovanni BR, Paolo Z, Sandro M, Paolo M, Riccardo A, Giovanni F, et al. A randomized trial of adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 versus standard therapy in the postoperative treatment of resected non-small cell lung carcinoma. Cancer 1996;78(2):244-51. - PubMed
Katakami 2017 {published data only}
-
- Katakami N, Hida T, Nokihara H, Imamura F, Sakai H, Atagi S, et al. Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non-small cell lung cancer. Lung Cancer 2017;105:23-30. - PubMed
Macchiarini 1991 {published data only}
-
- Macchiarini P, Hardin M, Angeletti CA. Long-term evaluation of intrapleural Bacillus Calmette-Guérin with or without adjuvant chemotherapy in completely resected stages II and III non-small-cell lung cancer. American Journal of Clinical Oncology 1991;14(4):291-7. - PubMed
Matthay 1986 {published data only}
-
- Matthay RA, Mahler DA, Beck GJ, Loke J, Baue AE, Carter DC, et al. Intratumoral Bacillus Calmette-Guérin immunotherapy prior to surgery for carcinoma of the lung: results of a prospective randomized trial. Cancer Research 1986;46(11):5963-8. - PubMed
Multhoff 2020 {published data only}EudraCT2008‐002130‐30
-
- Multhoff G, Seier S, Stangl S, Sievert W, Shevtsov M, Werner C, et al. Targeted natural killer cell-based adoptive immunotherapy for the treatment of patients with NSCLC after radiochemotherapy: a randomized phase II clinical trial. Clinical Trials: Immunotherapy 2020;26:5368-79. - PubMed
Stanley 1986 {published data only}
-
- Stanley K, Ludwig Lung Cancer Study Group (LLCSG). Immunostimulation with intrapleural BCG as adjuvant therapy in resected non-small cell lung cancer. Cancer 1986;58(11):2411-6. - PubMed
Vansteenkiste 2013 {published data only}
-
- Vansteenkiste J, Zielinski M, Linder A, Dahabreh J, Gonzalez EE, Malinowski W, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. Journal of Clinical Oncology 2013;31(19):2396-403. - PubMed
Vansteenkiste 2016 {published data only}
-
- Vansteenkiste JF, Cho BC, Vanakesa T, Pas T, Zielinski M, Kim MS, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncology 2016;17(6):822-35. - PubMed
Zhao 2014 {published data only}
References to studies excluded from this review
Bradley 2020 {published data only}
-
- Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE et al. Long-term results of NRG oncology RTOG 0617: standard versus high-dose chemoradiotherapy with or without cetuximab for unresectable Stage III non-small-cell lung cancer. Journal of Clinical Oncology 2020 Mar 1;38(7):706-14. [PMID: ] - PMC - PubMed
Giaccone 2020 {published data only}
-
- Giaccone G, Felip E, Cobo M, Campelo RG, Denis F, Quoix E et al. Activity of OSE-2101 in HLA-A2+ non-small cell lung cancer (NSCLC) patients after failure to immune checkpoint inhibitors (ICI): step 1 results of phase III ATALANTE-1 randomised trial. Annals of Oncology 2020;31(4):S814-S815. [DOI: 10.1016/j.annonc.2020.08.1574] - DOI
Kimura 2015 {published data only}
-
- Kimura H, Matsui Y, Ishikawa A, Nakajima T, Yoshino M, Sakairi Y. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated killer T cells and dendritic cells in patients with resected primary lung cancer. Cancer Immunology, Immunotherapy 2015 Jan;64(1):51-9. [PMID: ] - PMC - PubMed
Kimura 2018 {published data only}
-
- Kimura H, Matsui Y, Ishikawa A, Nakajima T, Lizasa T. Randomized controlled phase III trial of adjuvant chemo-immunotherapy with activated cytotoxic T cells and dendritic cells from regional lymph nodes of patients with lung cancer. Cancer Immunology, Immunotherapy 2018 Aug;67(8):1231-8. [PMID: ] - PMC - PubMed
Lee 2017 {published data only}
-
- Lee JM, Lee MH, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, et al. Phase I trial of intratumoral injection of CCL21 gene-modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8(+) T-cell infiltration. Clinical Cancer Research 2017 Aug 15;23(16):4556-68. [PMID: ] - PMC - PubMed
Mignard 2018 {published data only}
-
- Mignard X, Antoine M, Moro-Sibilot D, Dayen C, Mennecier B, Gervais R et al. IoNESCO trial: Immune neoajuvant therapy in early stage non-small cell lung cancer. Revue des Maladies Respiratoires 2018 Nov;35(9):983-8. [PMID: ] - PubMed
Nokihara 2017 {published data only}
-
- Nokihara H, Lu S, Mok TSK, Nakagawa K, Yamamoto N, Shi Y et al. Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Annals of Oncology 2017 Nov 1;28(11):2698-2706. [PMID: ] - PMC - PubMed
Rittmeyer 2017 {published data only}
-
- Rittmeyer, A Barlesi, F Waterkamp, D Park, K Ciardiello, F von Pawel, J et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017 Jan 21;389(10066):255-65. [PMID: ] - PMC - PubMed
References to ongoing studies
Merck 2015 {unpublished data only}
-
- NCT00960115. Study of Tecemotide (L-BLP25) in Participants With Stage III Unresectable Non-small Cell Lung Cancer (NSCLC) Following Primary Chemoradiotherapy. www.clinicaltrials.gov/ct2/show/NCT00960115 (first received 17 August 2009).
Saka 2017 {published data only}
-
- Saka H, Kitagawa C, Ichinose Y, Takenoyama M, Ibata H, Kato T, et al. A randomized phase II study to assess the effect of adjuvant immunotherapy using α-GalCer-pulsed dendritic cells in the patients with completely resected stage II–IIIA non-small cell lung cancer: study protocol for a randomized controlled trial. Trials 2017;18:429. - PMC - PubMed
Additional references
Aaronson 1993
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365-76. [PMID: ] - PubMed
Acres 2005
-
- Acres B, Limacher JM. MUC1 as a target antigen for cancer immunotherapy. Expert Review of Vaccines 2005;4(4):493-502. - PubMed
Aitken 1969
Antonia 2017
-
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. New England Journal of Medicine 2017;377(20):1919-29. [PMID: ] - PubMed
Bafna 2010
Borenstein 2008
-
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis (Statistics in Practice). Chichester: John Wiley & Sons, 2008.
Carrizosa 2015
Dasanu 2012
-
- Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert Opinion on Biological Therapy 2012;12(7):923-37. - PubMed
Detterbeck 2009
-
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136(1):260-71. - PubMed
Drake 2006
-
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Advances in Immunology 2006;90:51-81. - PubMed
Drake 2014
EuroQol 1990
-
- EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990 Dec;16(3):199-208. [PMID: ] - PubMed
Finn 2008
-
- Finn OJ. Cancer immunology. New England Journal of Medicine 2008;358(25):2704-15. - PubMed
Forde 2014
-
- Forde PM, Kelly RJ, Brahmer JR. New strategies in lung cancer: translating immunotherapy into clinical practice. Clinical Cancer Research 2014;20(5):1067-73. - PubMed
Giaccone 2013
-
- Giaccone G, Bazhenova L, Nemunaitis J, Juhasz E, Ramlau R, Van Den Heuval MM, et al. A phase III study of belagenpumatucel-L therapeutic tumor cell vaccine for non-small cell lung cancer (NSCLC). In: European Cancer Congress 2013. Amsterdam, 2013:Abstract n2.
GLOBOCAN 2020
-
- International Agency for Research on Cancer (IARC) . Cancer incidence and mortality worldwide. https://gco.iarc.fr/databases.php (accessed 8 November 2021).
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro. Version accessed prior to 1 November 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
Hayes 2021
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. - PMC - PubMed
Hirschowitz 2006
-
- Hirschowitz EA, Hiestand DM, Yannelli JR. Vaccines for lung cancer. Journal of Thoracic Oncology 2006;1(1):93-104. - PubMed
Howington 2013
-
- Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl):e278S-e313S. - PubMed
Keir 2008
Kochenderfer 2007
-
- Kochenderfer JN, Gress RE. A comparison and critical analysis of preclinical anticancer vaccination strategies. Experimental Biology and Medicine 2007;232(9):1130-41. - PubMed
Lardinois 2005
-
- Lardinois D, Suter H, Hakki H, Rousson V, Betticher D, Ris HB. Morbidity, survival, and site of recurrence after mediastinal lymph-node dissection versus systematic sampling after complete resection for non-small cell lung cancer. Annals of Thoracic Surgery 2005;80(1):268-74. - PubMed
Liberati 2009
Mellman 2011
Mountain 1997
-
- Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111:1710–17. - PubMed
National Cancer Institute 2017
-
- National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. www.seer.cancer.gov/statfacts/html/lungb.html (accessed 14 November 2017).
NCI‐CTCAE 2017
-
- National Cancer Institute (NCI). Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs... November 27, 2017.
NCT02273375
-
- NCT02273375. A phase III prospective double-blind placebo controlled randomized study of adjuvant MEDI4736 in completely resected non-small cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02273375.
NCT02486718
-
- NCT02486718. Study to assess safety and efficacy of atezolizumab (MPDL3280A) compared to best supportive care following chemotherapy in patients with lung cancer [IMpower010]. https://clinicaltrials.gov/ct2/show/NCT02486718.
NCT02504372
-
- NCT02504372. Study of pembrolizumab (MK-3475) vs placebo for participants with non-small cell lung cancer after resection with or without standard adjuvant therapy (MK-3475-091/KEYNOTE-091) (PEARLS). https://clinicaltrials.gov/ct2/show/NCT02504372.
Nemunaitis 2006
-
- Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. Journal of Clinical Oncology 2006;24(29):4721-30. - PubMed
Peters 2008
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991–6. - PubMed
Ramnath 2013
-
- Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl):e314S-e40S. - PubMed
Reckamp 2015
-
- Reckamp KL. Advances in immunotherapy for non-small cell lung cancer. Clinical Advances in Hematology & Oncology 2015;13(12):847-53. - PubMed
Review Manager 2020 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Roy 2008
Sangha 2007
-
- Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clinical Cancer Research 2007;13(15 Pt 2):s4652-4. - PubMed
Simpson 2005
-
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nature Reviews 2005;5(8):615-25. - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada, National Cancer Institute of the United States. Journal of the National Cancer Institute 2000;92(3):205-16. - PubMed
Topalian 2012
Tyagi 2009
-
- Tyagi P, Mirakhur B. MAGRIT: the largest-ever phase III lung cancer trial aims to establish a novel tumor-specific approach to therapy. Clinical Lung Cancer 2009;10(5):371-4. - PubMed
Van den Eynde 1997
-
- Van den Eynde BJ, Bruggen P. T cell defined tumor antigens. Current Opinion in Immunology 1997;9(5):684-93. - PubMed
Wolchok 2009
-
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clinical Cancer Research 2009;15(23):7412-20. - PubMed
References to other published versions of this review
Song 2014
-
- Song H, Zhu J, Suo C, Lu D. Immunotherapy for stage I‐III non‐small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD011300. [DOI: 10.1002/14651858.CD011300] - DOI
Zhu 2017
-
- Zhu J, Li R, Tiselius E, Roudi R, Teghararian O, Suo C et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database of Systematic Reviews 2017 Dec 16;12(12):CD011300. [PMID: ] - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

