Analyzing Patient Trajectories With Artificial Intelligence
- PMID: 34870606
- PMCID: PMC8686456
- DOI: 10.2196/29812
Analyzing Patient Trajectories With Artificial Intelligence
Abstract
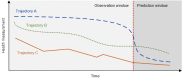
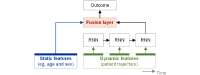
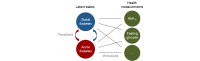
In digital medicine, patient data typically record health events over time (eg, through electronic health records, wearables, or other sensing technologies) and thus form unique patient trajectories. Patient trajectories are highly predictive of the future course of diseases and therefore facilitate effective care. However, digital medicine often uses only limited patient data, consisting of health events from only a single or small number of time points while ignoring additional information encoded in patient trajectories. To analyze such rich longitudinal data, new artificial intelligence (AI) solutions are needed. In this paper, we provide an overview of the recent efforts to develop trajectory-aware AI solutions and provide suggestions for future directions. Specifically, we examine the implications for developing disease models from patient trajectories along the typical workflow in AI: problem definition, data processing, modeling, evaluation, and interpretation. We conclude with a discussion of how such AI solutions will allow the field to build robust models for personalized risk scoring, subtyping, and disease pathway discovery.
Keywords: artificial intelligence; digital medicine; longitudinal data; machine learning; patient trajectories.
©Ahmed Allam, Stefan Feuerriegel, Michael Rebhan, Michael Krauthammer. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 03.12.2021.
Conflict of interest statement
Conflicts of Interest: MR declares employment with the Novartis Institutes for Biomedical Research, Switzerland.
Figures

References
-
- Jensen AB, Moseley PL, Oprea TI, Ellesøe SG, Eriksson R, Schmock H, Jensen PB, Jensen LJ, Brunak S. Temporal disease trajectories condensed from population-wide registry data covering 6.2 million patients. Nat Commun. 2014 Jun 24;5(1):4022. doi: 10.1038/ncomms5022. doi: 10.1038/ncomms5022.ncomms5022 - DOI - DOI - PMC - PubMed
-
- Rajkomar A, Oren E, Chen K, Dai AM, Hajaj N, Hardt M, Liu PJ, Liu X, Marcus J, Sun M, Sundberg P, Yee H, Zhang K, Zhang Y, Flores G, Duggan GE, Irvine J, Le Q, Litsch K, Mossin A, Tansuwan J, Wang D, Wexler J, Wilson J, Ludwig D, Volchenboum SL, Chou K, Pearson M, Madabushi S, Shah NH, Butte AJ, Howell MD, Cui C, Corrado GS, Dean J. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018 May 8;1(1):18. doi: 10.1038/s41746-018-0029-1. doi: 10.1038/s41746-018-0029-1.29 - DOI - DOI - PMC - PubMed
-
- Xiao C, Choi E, Sun J. Opportunities and challenges in developing deep learning models using electronic health records data: a systematic review. J Am Med Inform Assoc. 2018 Oct 01;25(10):1419–28. doi: 10.1093/jamia/ocy068. http://europepmc.org/abstract/MED/29893864 5035024 - DOI - PMC - PubMed
-
- Woldaregay AZ, Årsand E, Botsis T, Albers D, Mamykina L, Hartvigsen G. Data-driven blood glucose pattern classification and anomalies detection: machine-learning applications in type 1 diabetes. J Med Internet Res. 2019 May 01;21(5):e11030. doi: 10.2196/11030. https://www.jmir.org/2019/5/e11030/ v21i5e11030 - DOI - PMC - PubMed
-
- Noah B, Keller MS, Mosadeghi S, Stein L, Johl S, Delshad S, Tashjian VC, Lew D, Kwan JT, Jusufagic A, Spiegel BM. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digit Med. 2018 Jan 15;1(1):20172. doi: 10.1038/s41746-017-0002-4. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

