Altered protein secretion in Batten disease
- PMID: 34870700
- PMCID: PMC8669491
- DOI: 10.1242/dmm.049152
Altered protein secretion in Batten disease
Abstract
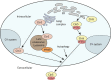
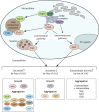
The neuronal ceroid lipofuscinoses (NCLs), collectively known as Batten disease, are a group of neurological diseases that affect all ages and ethnicities worldwide. There are 13 different subtypes of NCL, each caused by a mutation in a distinct gene. The NCLs are characterized by the accumulation of undigestible lipids and proteins in various cell types. This leads to progressive neurodegeneration and clinical symptoms including vision loss, progressive motor and cognitive decline, seizures, and premature death. These diseases have commonly been characterized by lysosomal defects leading to the accumulation of undigestible material but further research on the NCLs suggests that altered protein secretion may also play an important role. This has been strengthened by recent work in biomedical model organisms, including Dictyostelium discoideum, mice, and sheep. Research in D. discoideum has reported the extracellular localization of some NCL-related proteins and the effects of NCL-related gene loss on protein secretion during unicellular growth and multicellular development. Aberrant protein secretion has also been observed in mammalian models of NCL, which has allowed examination of patient-derived cerebrospinal fluid and urine for potential diagnostic and prognostic biomarkers. Accumulated evidence links seven of the 13 known NCL-related genes to protein secretion, suggesting that altered secretion is a common hallmark of multiple NCL subtypes. This Review highlights the impact of altered protein secretion in the NCLs, identifies potential biomarkers of interest and suggests that future work in this area can provide new therapeutic insight.
Keywords: Dictyostelium discoideum; Batten disease; Cerebrospinal fluid; Model system; Neuronal ceroid lipofuscinosis; Secretion; Urine.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The author declares no competing or financial interests.
Figures

References
-
- Adams, J., Feuerborn, M., Molina, J. A., Wilden, A. R., Adhikari, B., Budden, T. and Lee, S. Y. (2019). Autophagy-lysosome pathway alterations and alpha-synuclein up-regulation in the subtype of neuronal ceroid lipofuscinosis, CLN5 disease. Sci. Rep. 9, 151. 10.1038/s41598-018-36379-z - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

